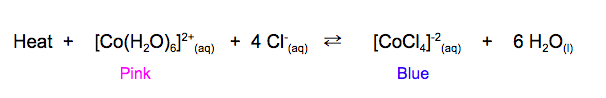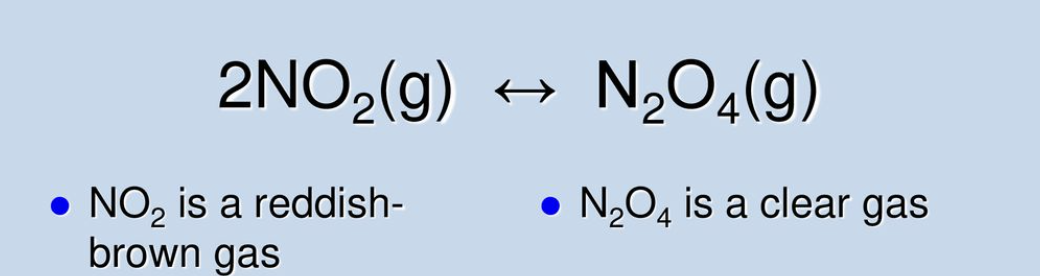Le Chat's Lab Review
star
star
star
star
star
Last updated about 1 month ago
17 questions
Le Chatelier Lab Practice
Required
1
Required
1
Required
1
Required
1
Required
1
Temperature and Le Chat's
Introduction to Le Chatelier and Changes in Volume/ Gas Pressure
Shifting as a result of changes in volume/pressure
Reflection
Required
1