Human activity affects cycling of nitrogen.
We humans may not be able to fix nitrogen biologically, but we certainly do industrially! About 450 million metric tons of fixed nitrogen are made each year using a chemical method called the Haber-Bosch process, in which N2, is reacted with hydrogen H2—at high temperatures. Most of this fixed nitrogen goes to make fertilizers we use on our lawns, gardens, and agricultural fields.
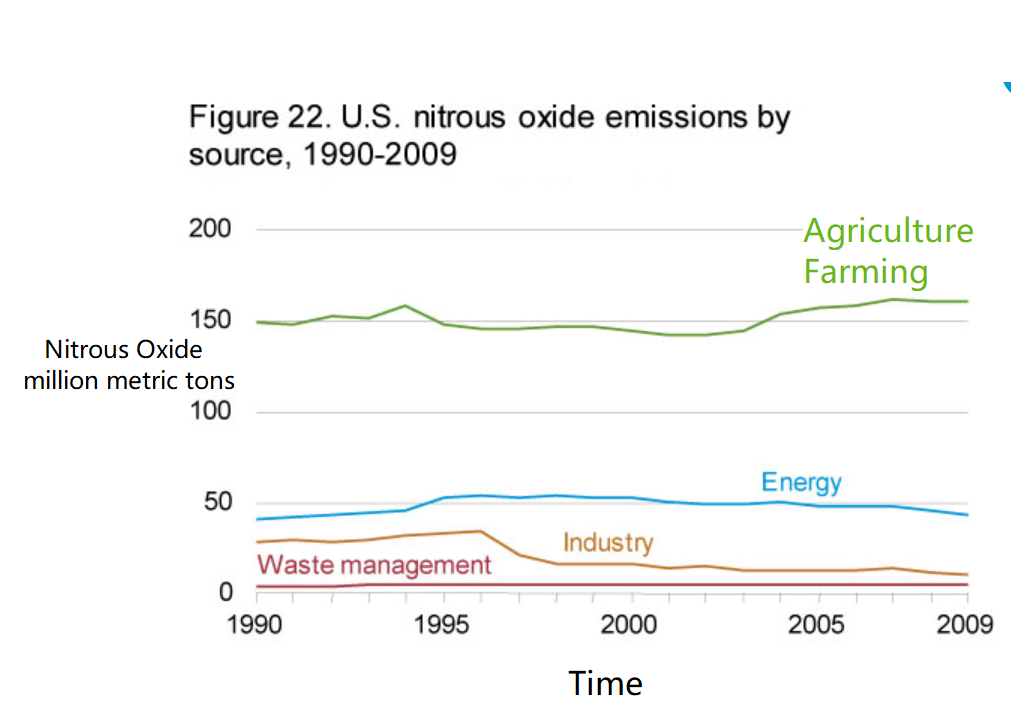
In general, human activity releases nitrogen into the environment by two main means: combustion of fossil fuels and use of nitrogen-containing fertilizers in agriculture. Both processes increase levels of nitrogen-containing compounds in the atmosphere. High levels of atmospheric nitrogen—other than N2—are associated with harmful effects, like the production of acid rain—as nitric acid, HNO3—and contributions to the greenhouse effect—as nitrous oxide, N2O.
Also, when artificial fertilizers containing nitrogen and phosphorous are used in agriculture, the excess fertilizer may be washed into lakes, streams, and rivers by surface runoff. A major effect from fertilizer runoff is saltwater and freshwater eutrophication. In this process, nutrient runoff causes overgrowth, or a "bloom," of algae or other microorganisms. Without the nutrient runoff, they were limited in their growth by availability of nitrogen or phosphorus.
Eutrophication can reduce oxygen availability in the water during the nighttime because the algae and microorganisms that feed on them use up large quantities of oxygen in cellular respiration. This can cause the death of other organisms living in the affected ecosystems, such as fish and shrimp, and result in low-oxygen, species-depleted areas called dead zones.