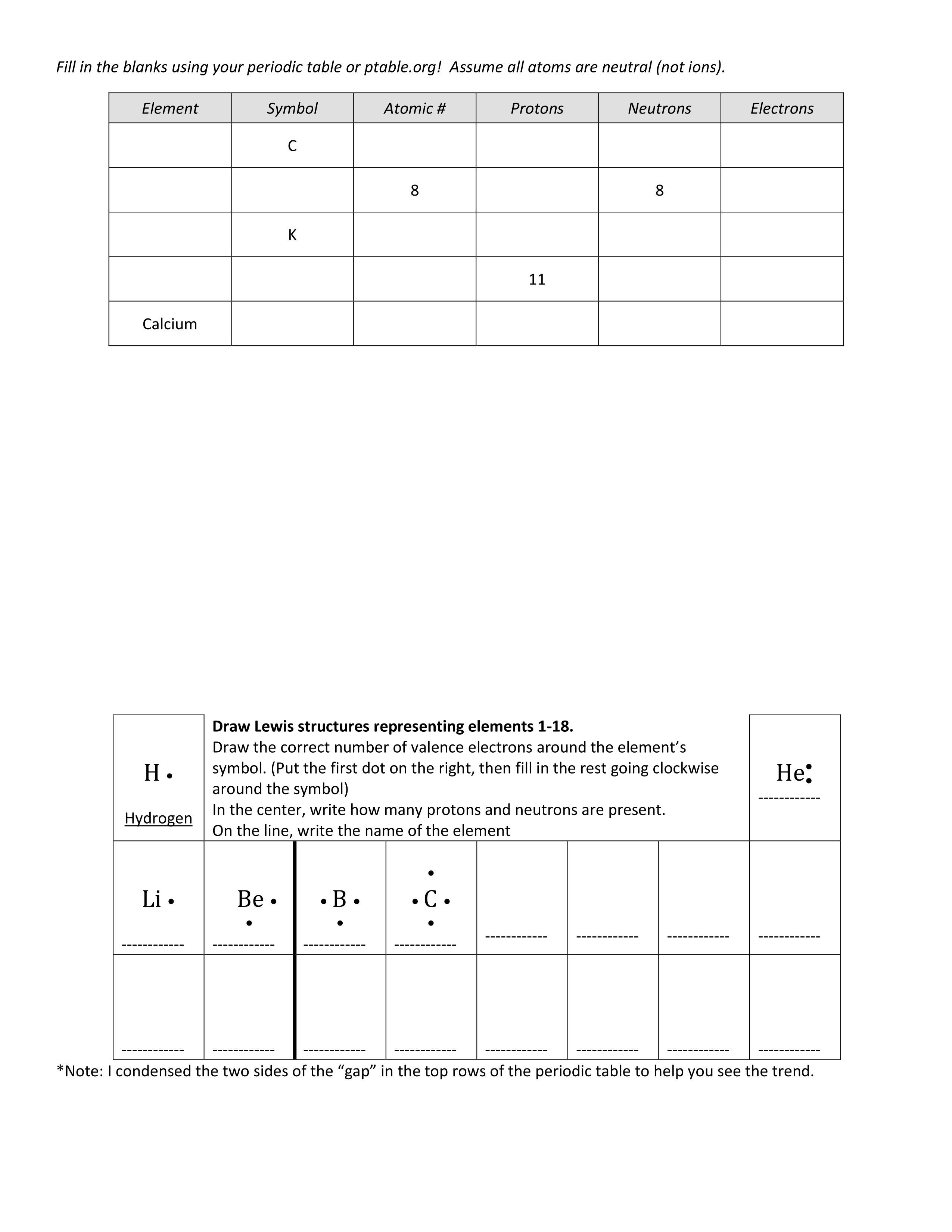
AGHS - Bio - Atomic Structure Worksheet
star
star
star
star
star
Last updated over 5 years ago
55 questions
1
1
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
15.9994 | arrow_right_alt | symbol |
O | arrow_right_alt | element name |
oxygen | arrow_right_alt | atomic mass |
8 | arrow_right_alt | atomic number |
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
atomic # = | arrow_right_alt | the number of protons |
# of protons = | arrow_right_alt | the # of electrons |
mass # = | arrow_right_alt | protons + neutrons |