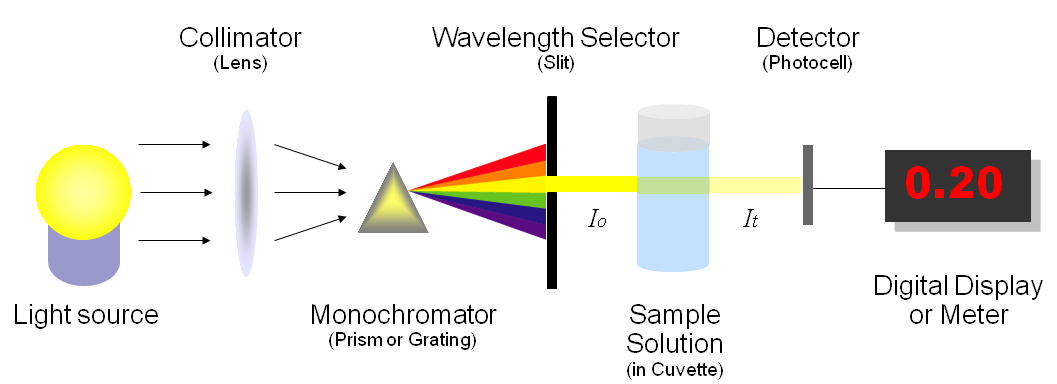
Beer's Law Simulation for AP Chemistry
star
star
star
star
star
Last updated over 5 years ago
24 questions
Note from the author:
Activity to accompany Beer's Law PhET. Adapted from activity by Brian Brown.
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1