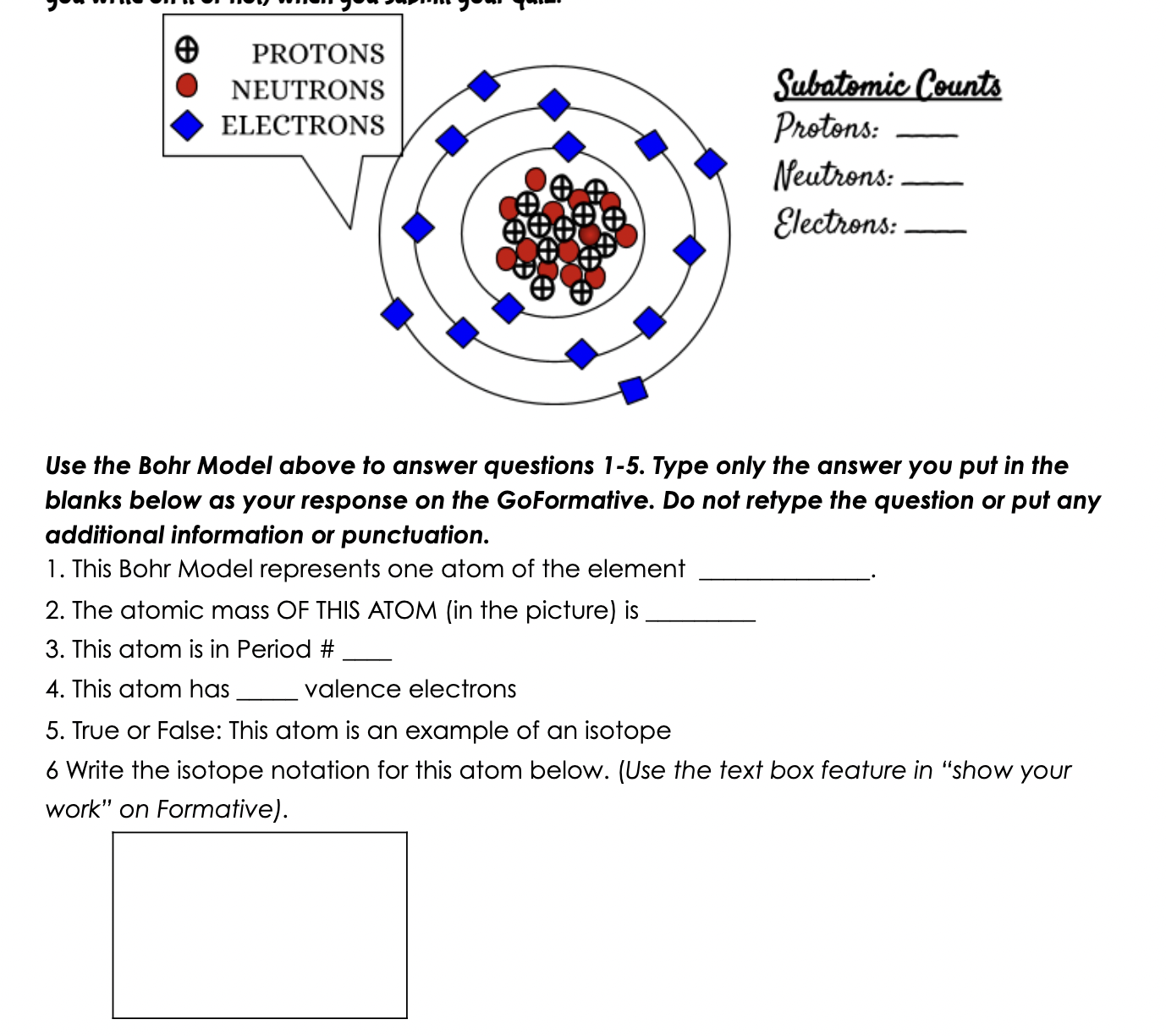
DIRECTIONS: Use the yellow periodic table on your test as a cover sheet for the paper you were given and a tool for the questions you will be asked. do not write on the periodic table.
There are several questions that might be difficult to see or do well via computer, these sections are on the piece of paper that was provided.
You may write on this paper. HOWEVER, please be careful not to show anyone the answers. You MUST turn this paper in (even if you don't use it/write on it) when you submit your quiz. There is an extra credit question on the paper.
When you are done: Make sure your name is on your paper and then turn it in UNDER the colored piece of paper in the white bin. Make sure your homework is completed for tomorrow (check the board).