Kelso High-Chem H-Reaction types
star
star
star
star
star
Last updated about 5 years ago
20 questions
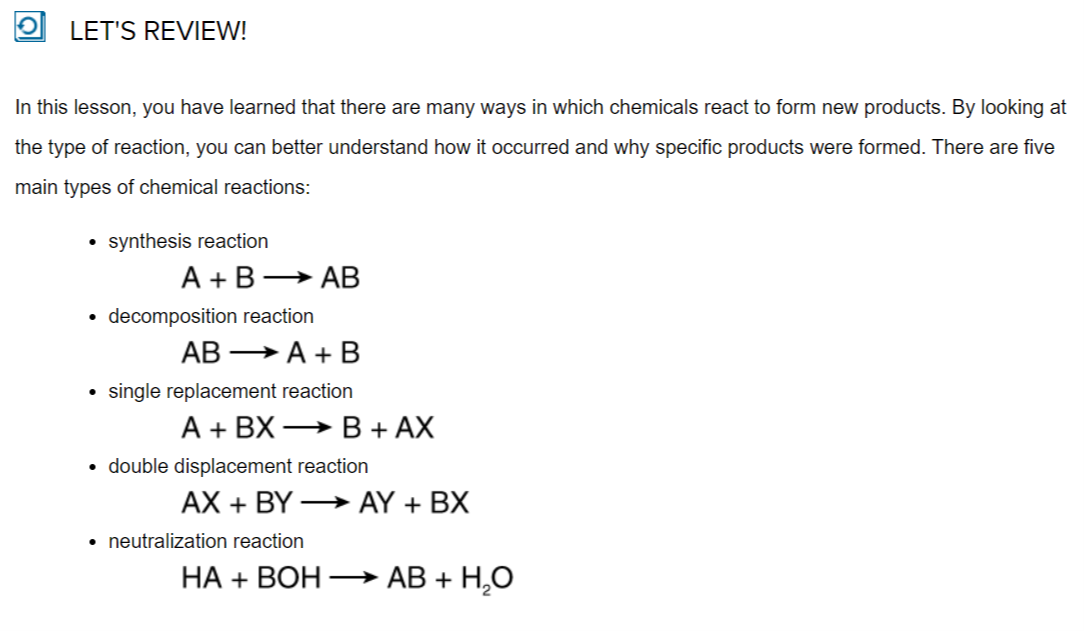
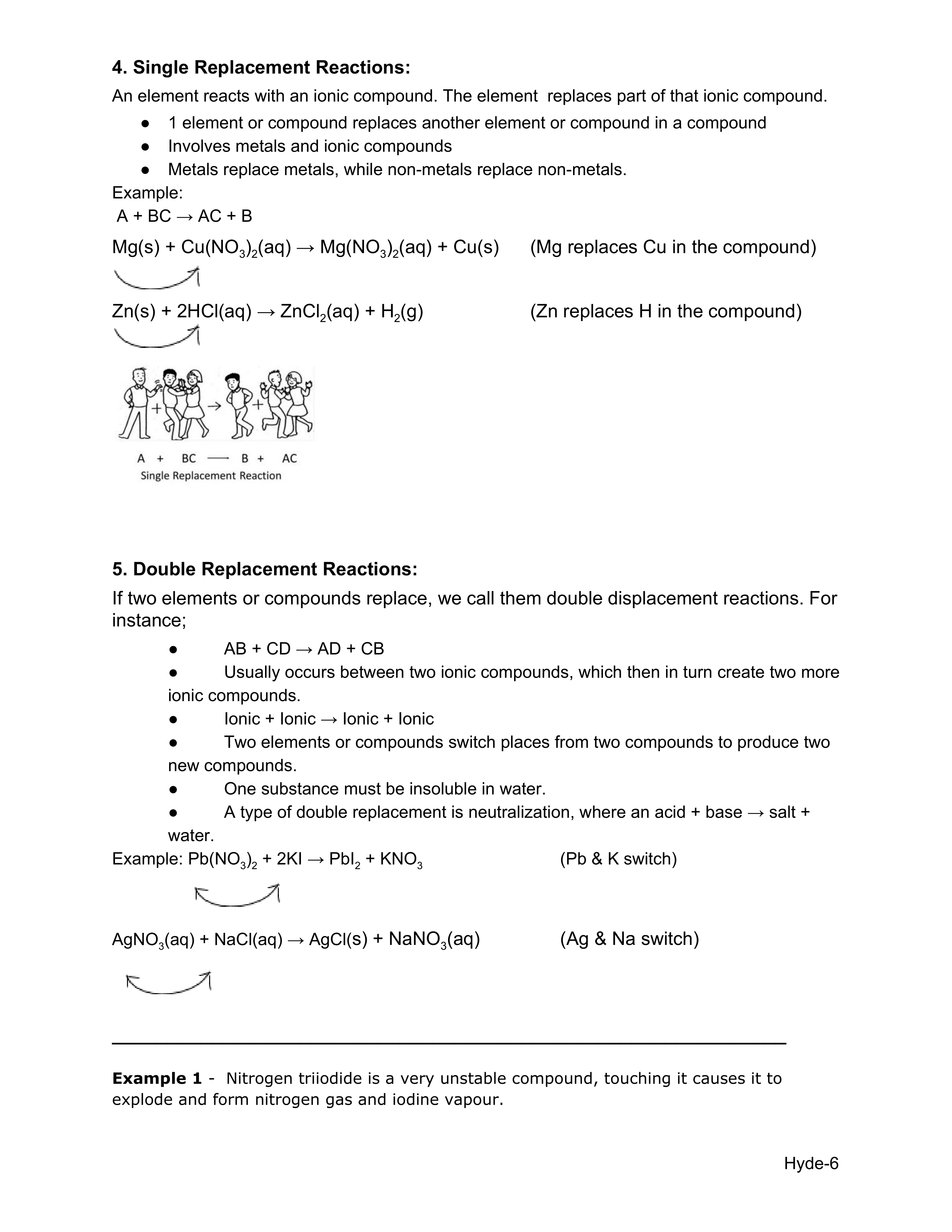
Why do sodas fizz when you first open them? And why does rust form on metal that is left outside? Both of these phenomena occur because of chemical reactions, but the way in which the reactions occur is different. There are actually several different types of chemical reactions. Reactants may combine, split apart, or replace other reactants to form the final products. In this lesson, you will learn to recognize five basic types of chemical reactions. Knowing how a given reaction works will help you understand what is happening and how specific products are being formed.

Now your turn: identify type of each reaction. Balance 1-5 only, do this in the show your work section.