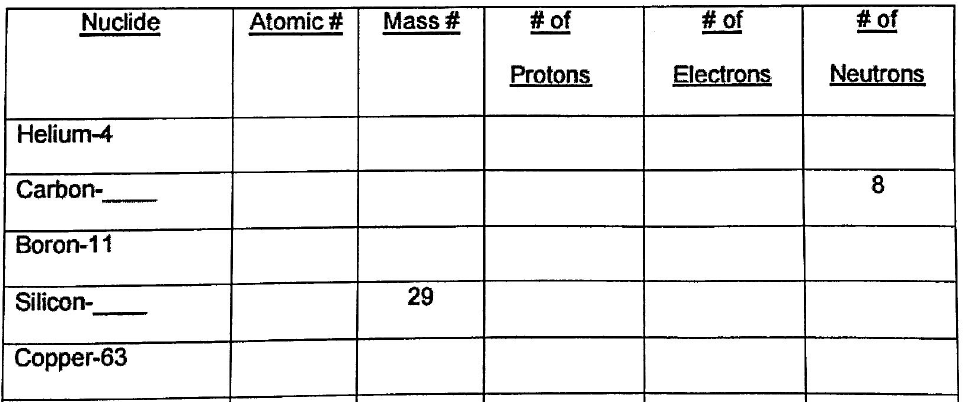
In the symbol portion of the simulation, play around with the particles until you can identify each part of the symbol, which particles (proton, neutron and/or electron) that affects that part and how the value of the number is determined. Use the text box to add the labels. The terms to label are mass number, atomic number, charge and symbol.