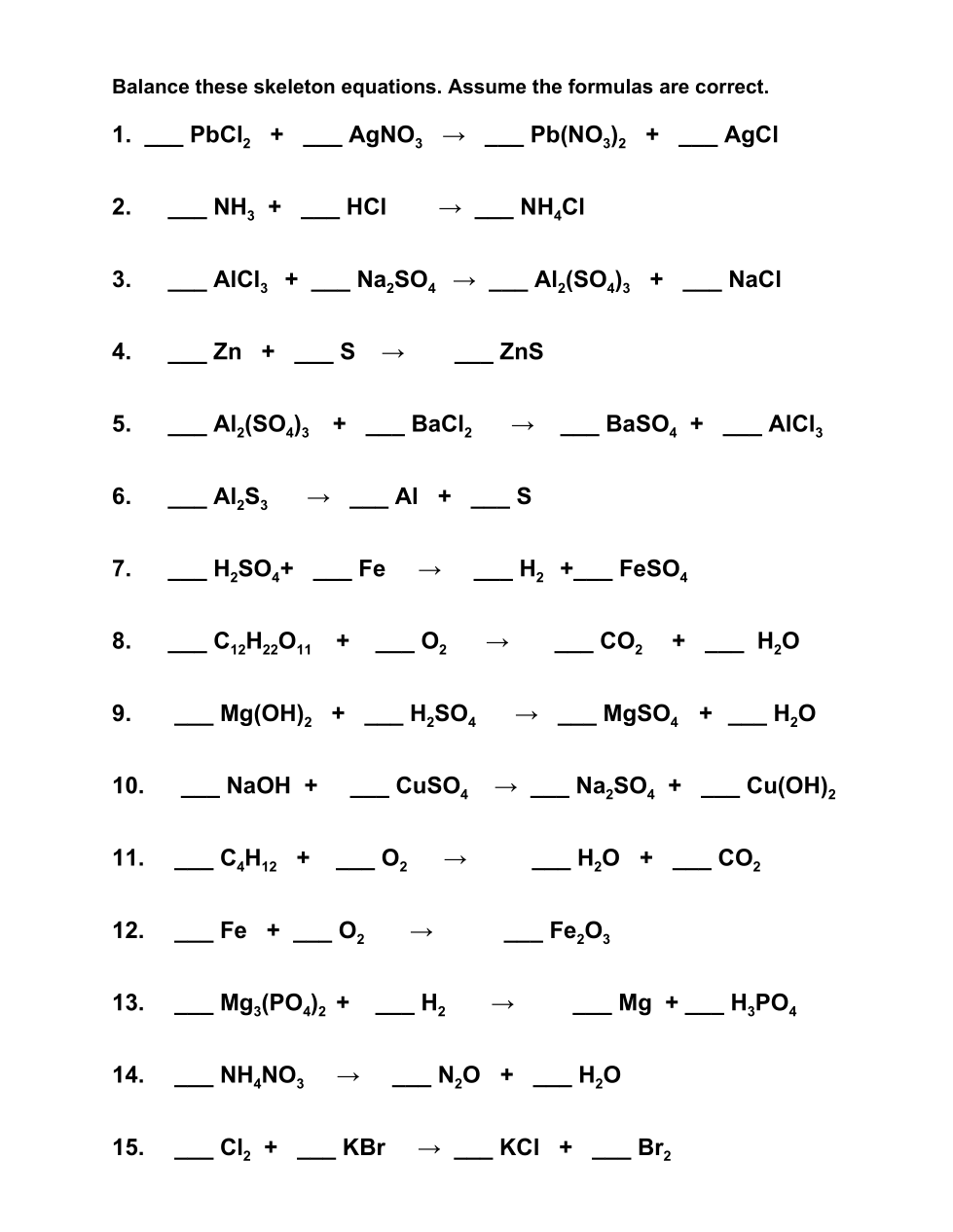
18 Balancing practice
star
star
star
star
star
Last updated over 4 years ago
15 questions
A coefficient is the number in front of the chemical symbol that indicates how many of that unit there are. Example: 3 AgCl means there are 3 Ag and 3 Cl , and each Ag is partnered with a Cl until they are dissolved in water.
Your job is to figure out how many groups of each substance in the chemical equation there has to be for the conservation of mass to be observed. The mass before the reaction equals the mass after the reaction
Enter the coefficients in the answer box like this: 1, 1, 2 or 2, 1, 2, 3 meaning, from left to right, coefficient of the first substance comma space coefficient of the 2nd substance comma space etcetera until all coefficients are shown. In this instance we do write the 1’s. If they are all ones, you may write “balanced”. There should be a number for each blank.