BONUS: Here are links to EXCELLENT video tutorials by topic:
Historical Development of Atom:
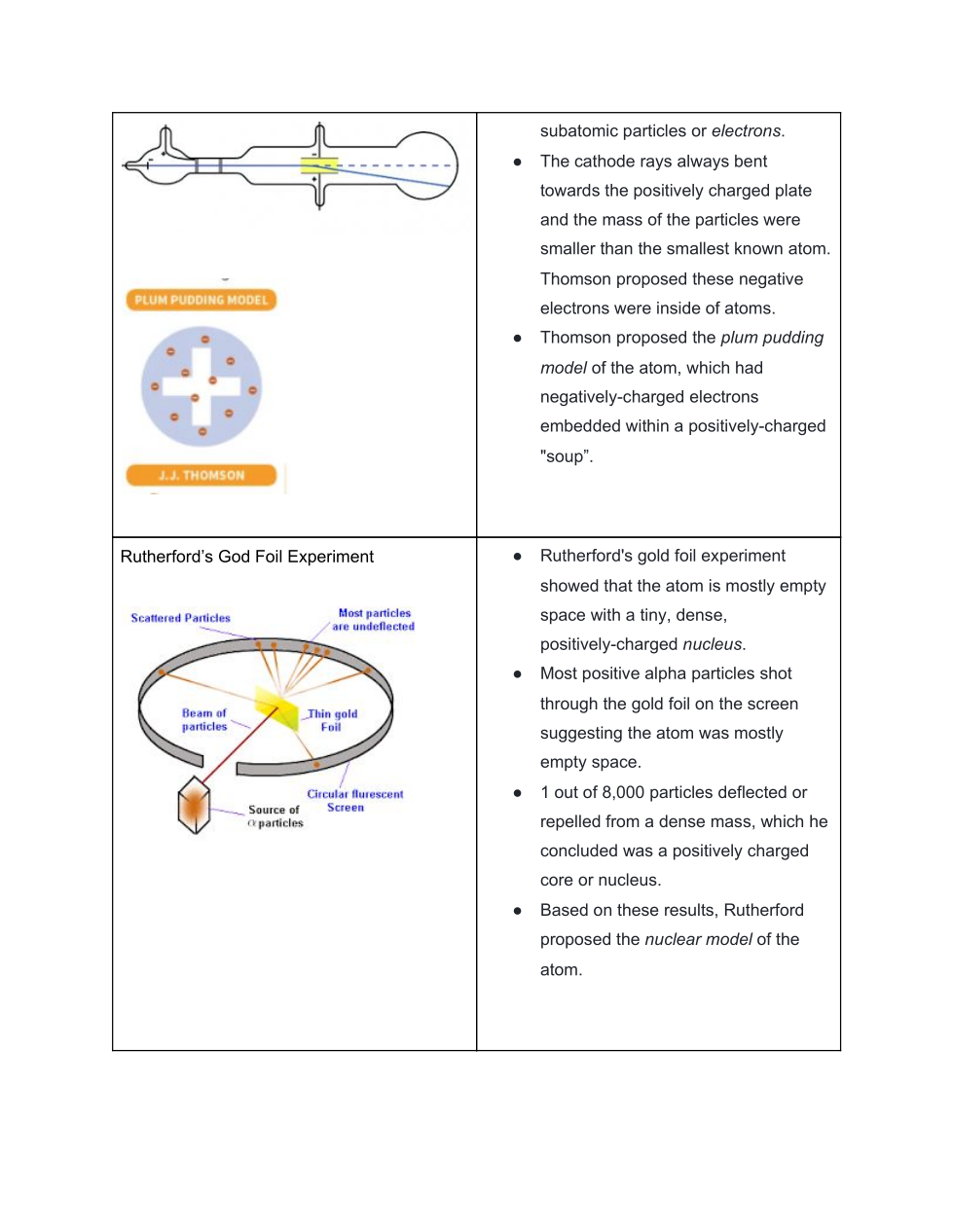
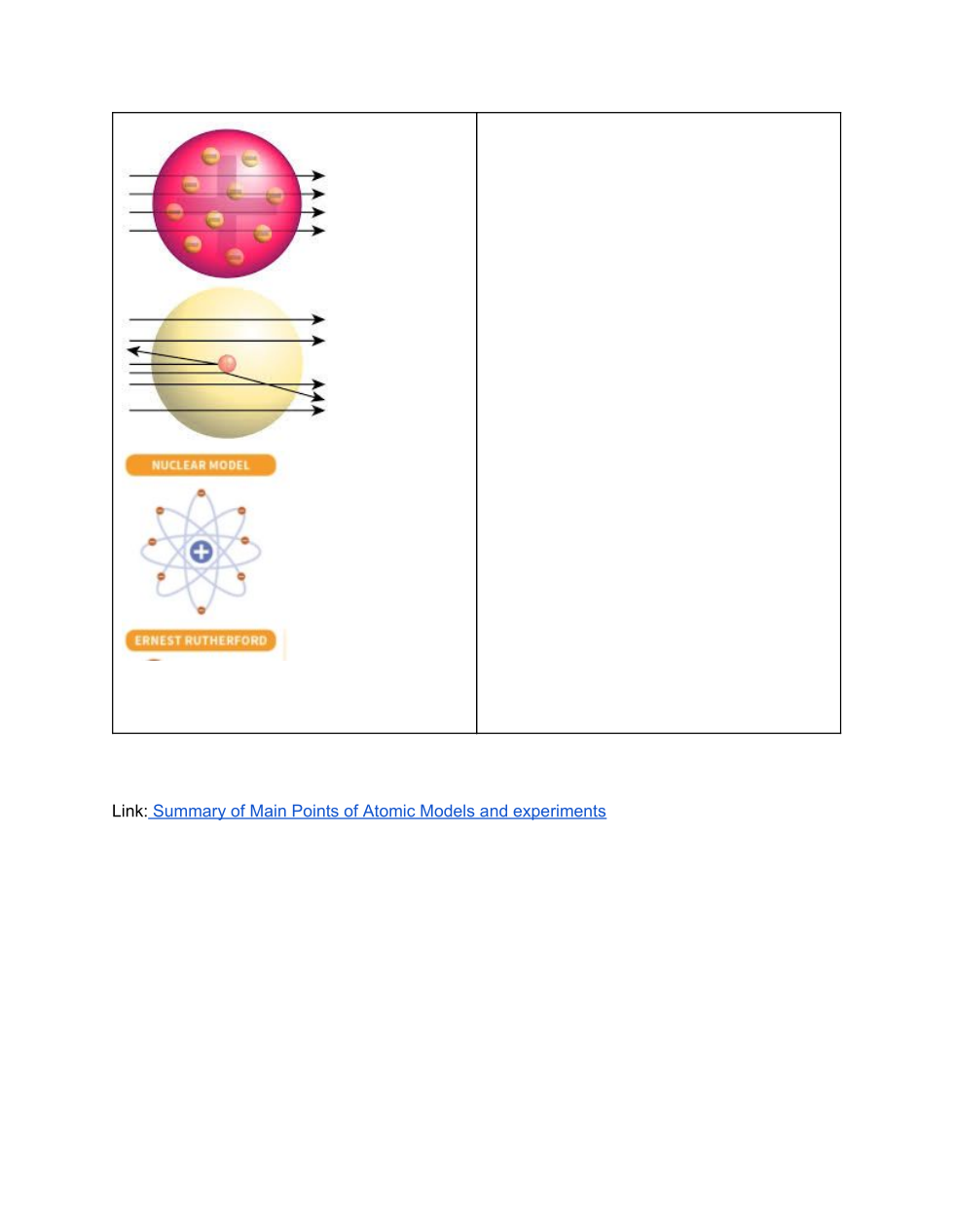
Rutherford’s Gold Foil Experiment
Wave Particle Duality: Double Slit Experiment
Wave Particle Duality: Photoelectric Effect
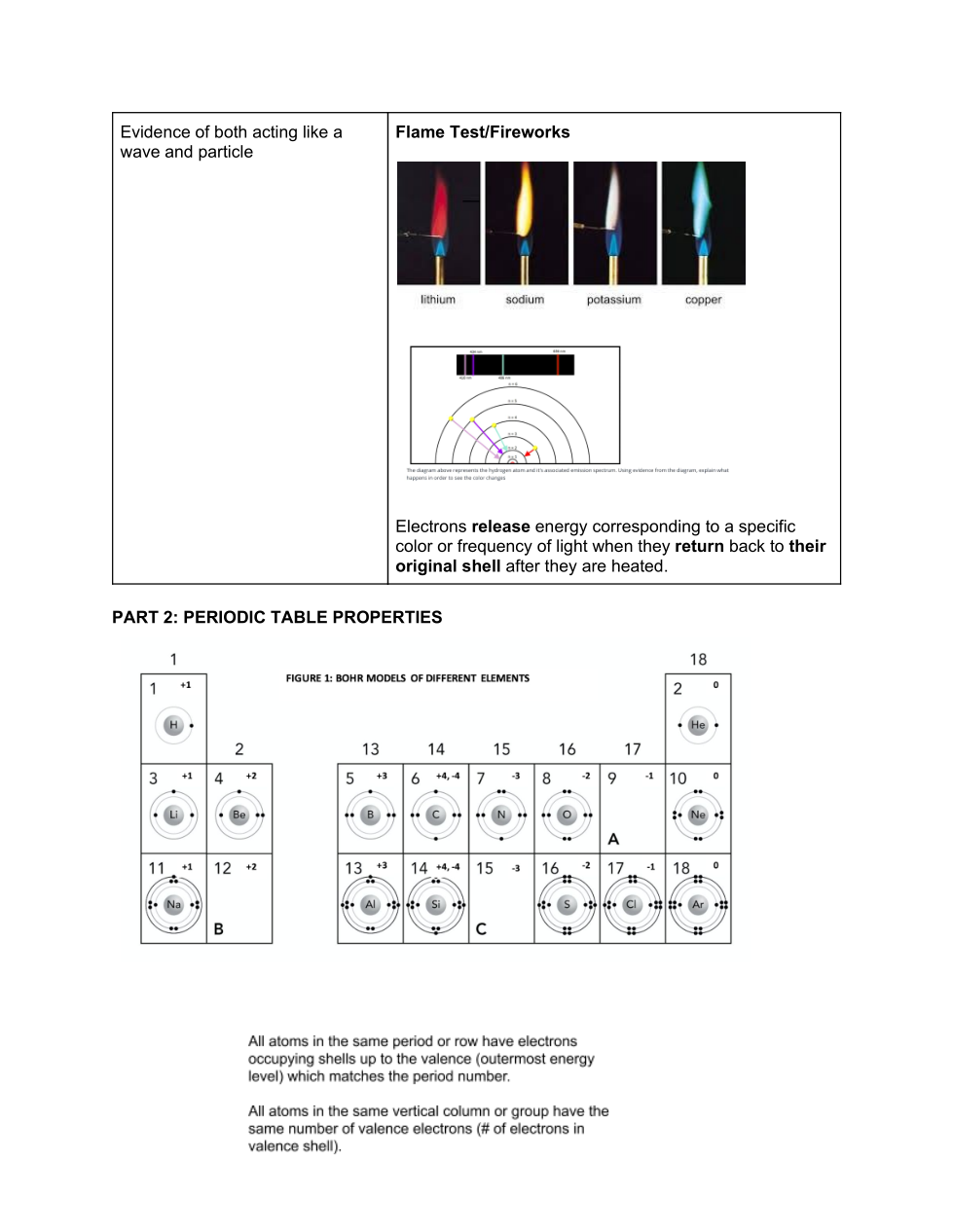
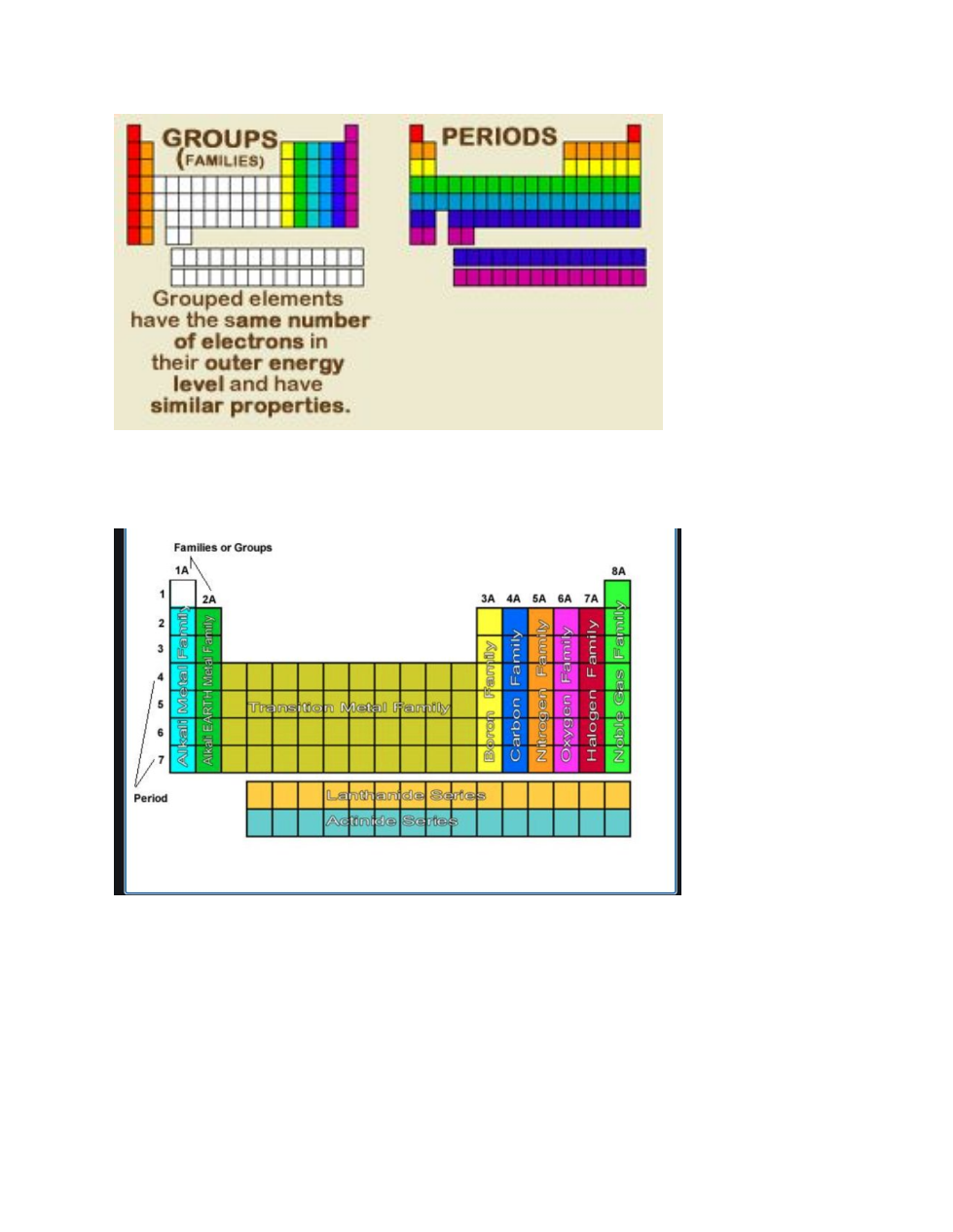
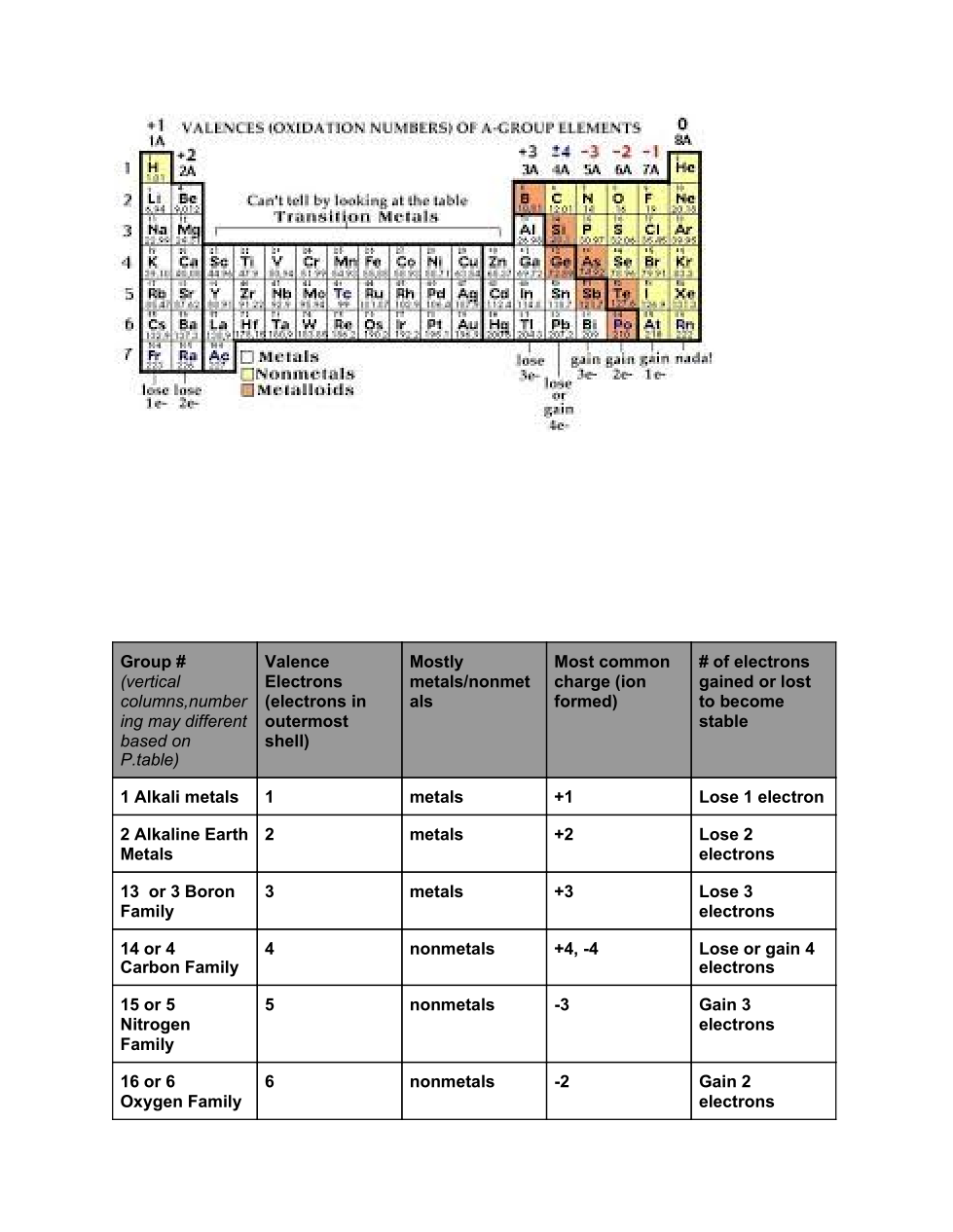
Periodic Table Properties:
Tour of the Periodic Table
Valence electrons and the Periodic table
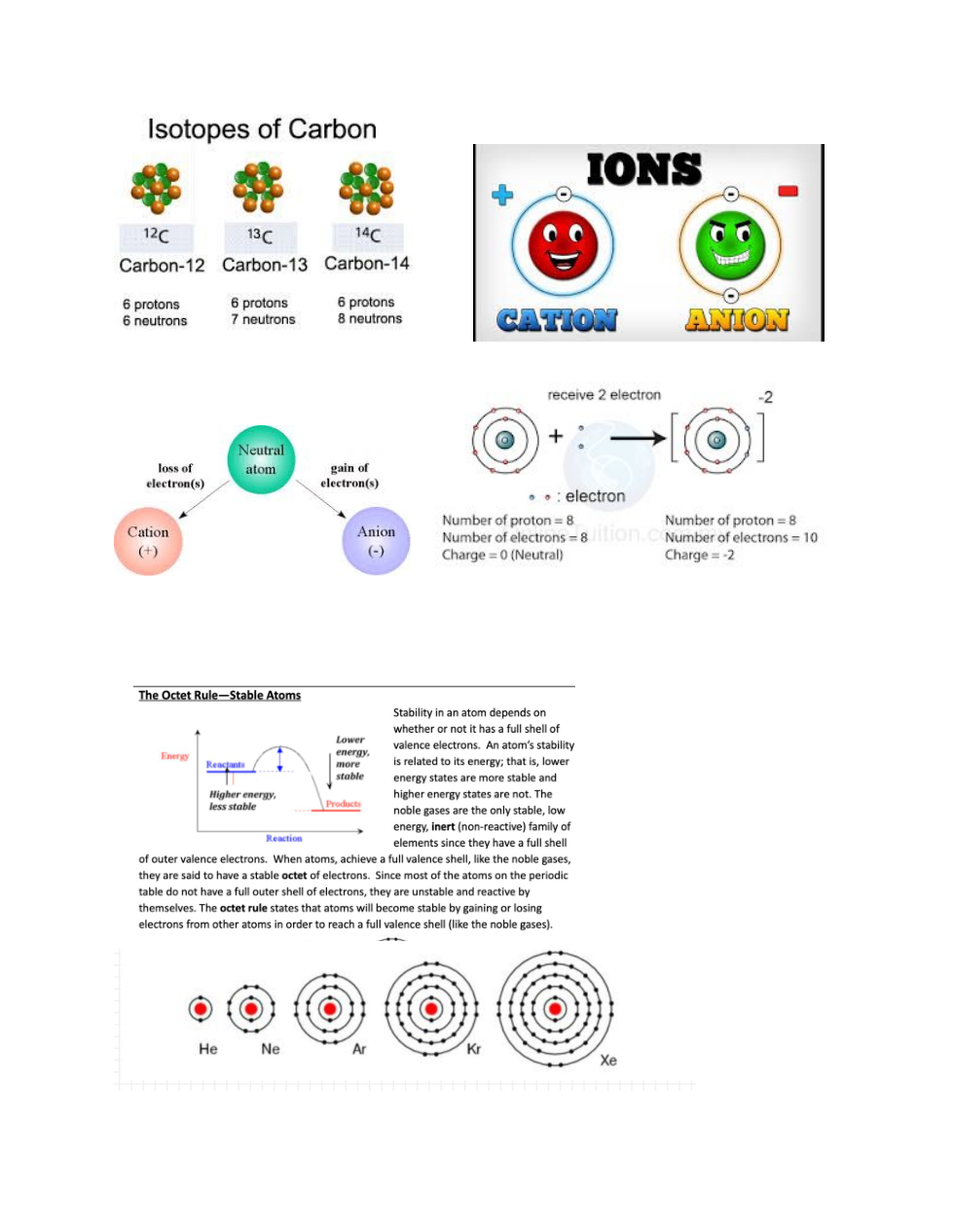
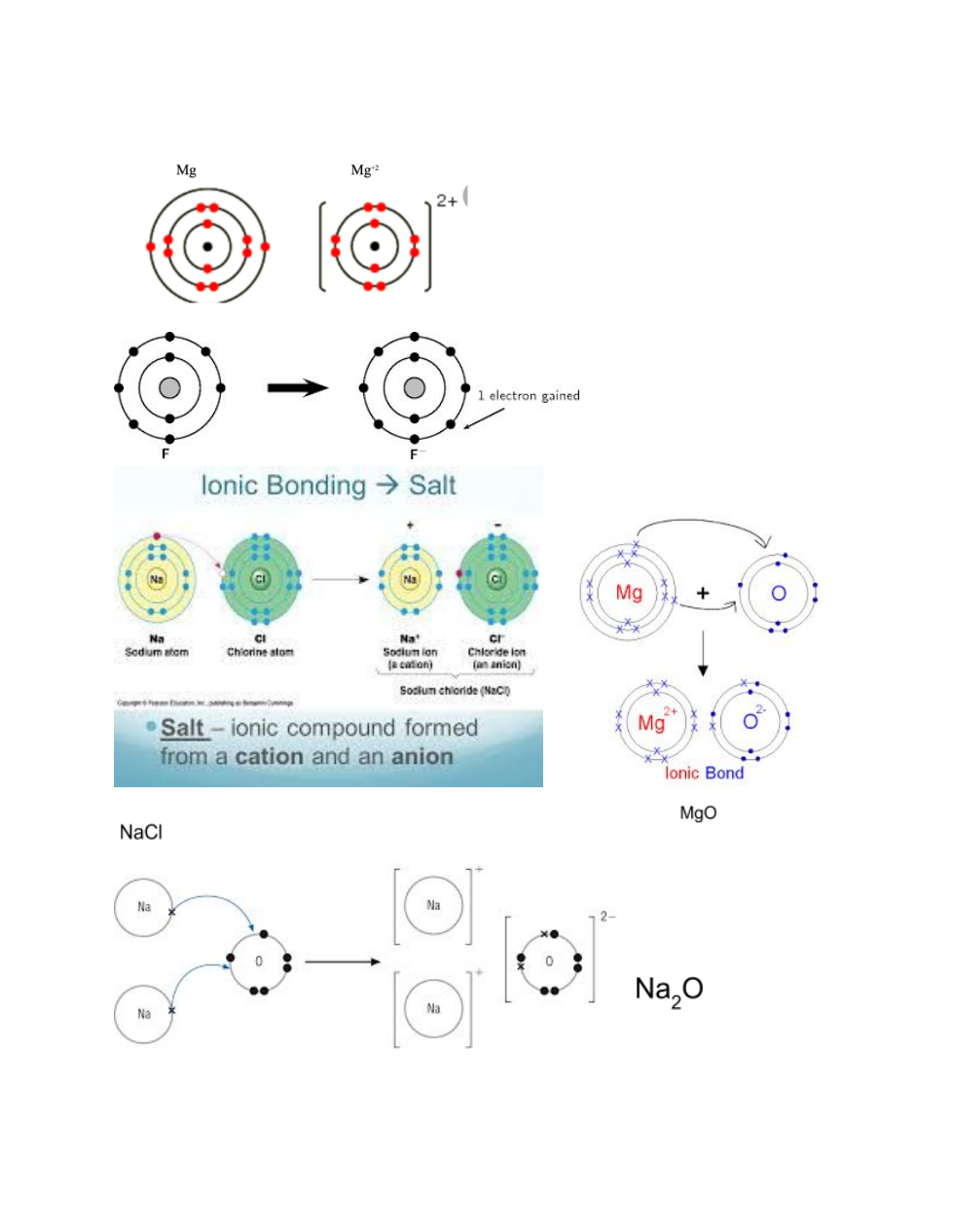
Atomic Structure and Ionic Bonding:
Atomic Number, Mass Number and Net Charge
Ionic Bonding: Video 1 Video 2