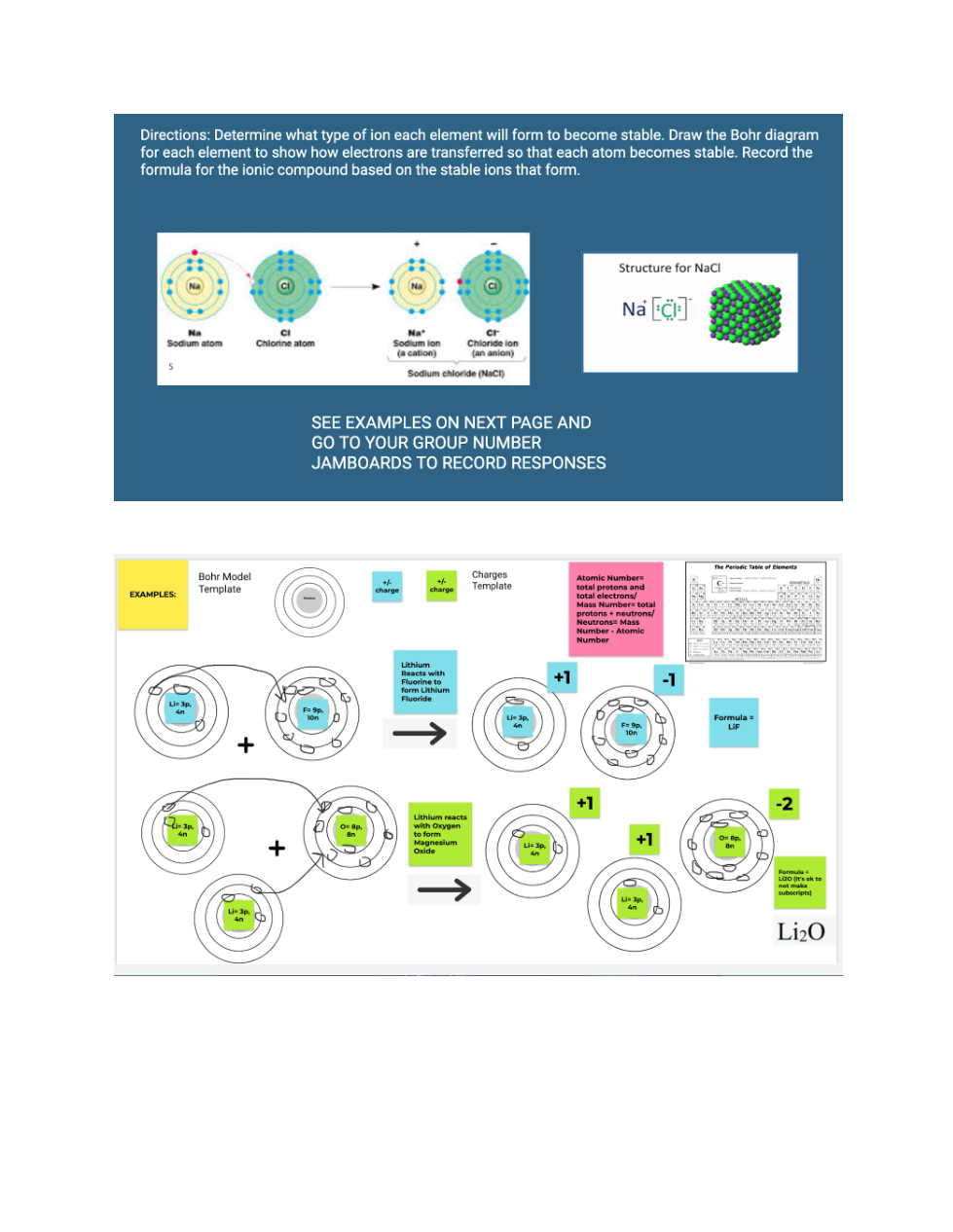
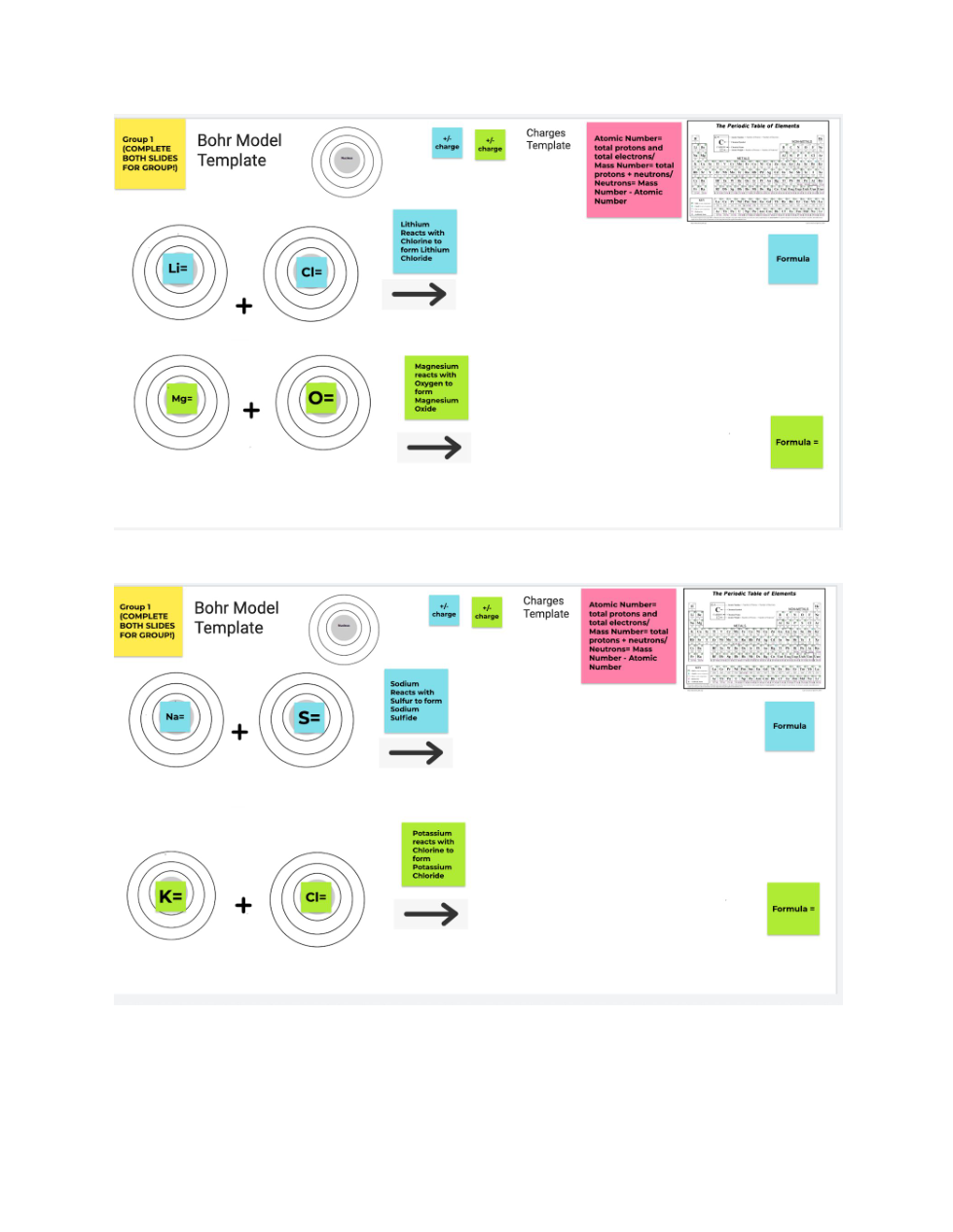
Zoom in to read the directions and view the examples given on the 1st page. The periodic table is given below. You'll need to zoom in to see elements better.
The atomic number= protons and total electrons
The mass number= protons + neutrons
Neutrons= Mass number - atomic number
The group number= valence electrons