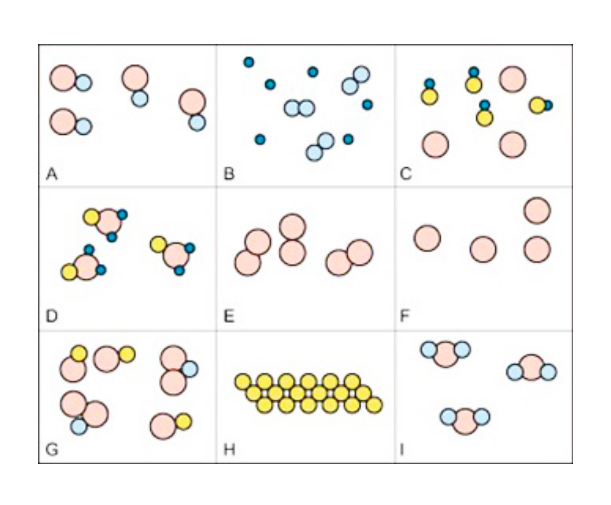
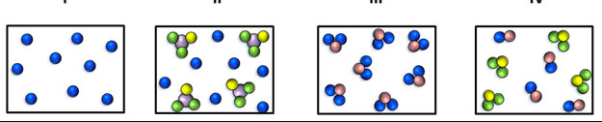
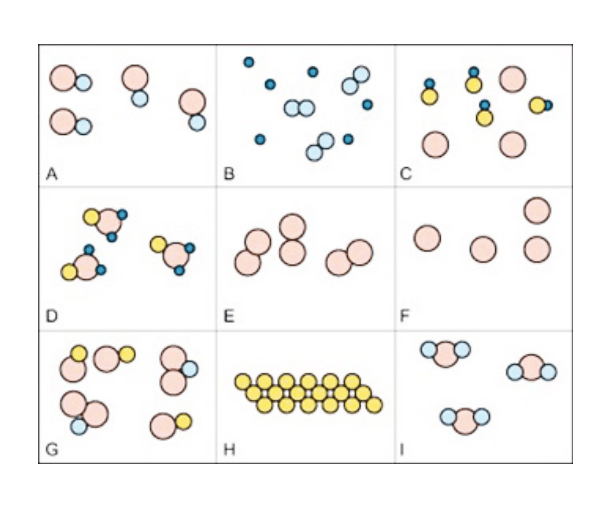
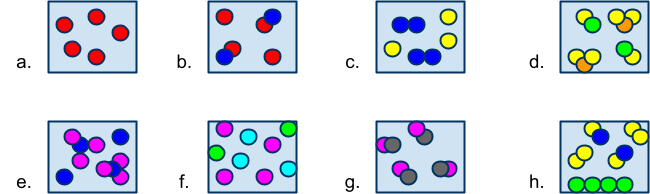
Classifying & Counting Matter Quiz
star
star
star
star
star
Last updated 9 months ago
38 questions
Untitled Section
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
0.5
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1