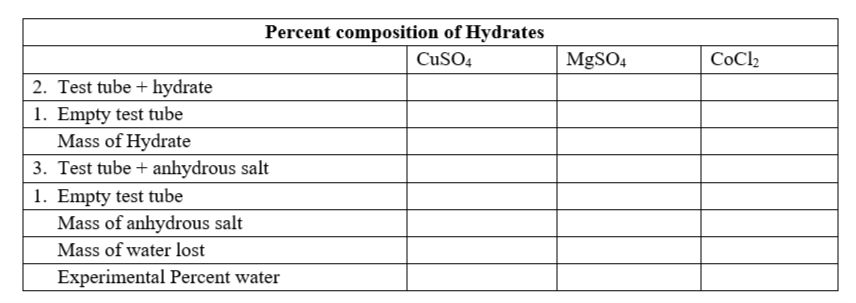
Remember to compare the ratio of moles, you calculate the moles of each substance to be compared. Then you divide by the smaller amount of moles. So determine the moles of the anhydrous salt and the moles of water contained in the sample. Divide by the smaller mole amount. The salt should be the smaller one. If not, there was an error in the procedure.