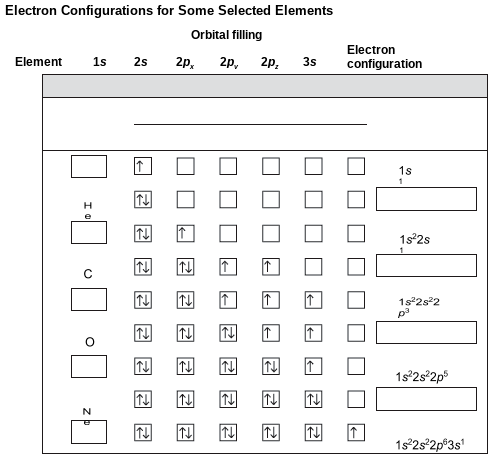
Each element atom has a unique configuration based on the number of electrons that matches the number of protons, or the atomic number. Use the filled-in information to determine what should be filled in for each marked area. For example, the last line has the diagram and configuration filled in. Each reflects 11 electrons. The element with 11 electrons is sodium so the answer would be Na.