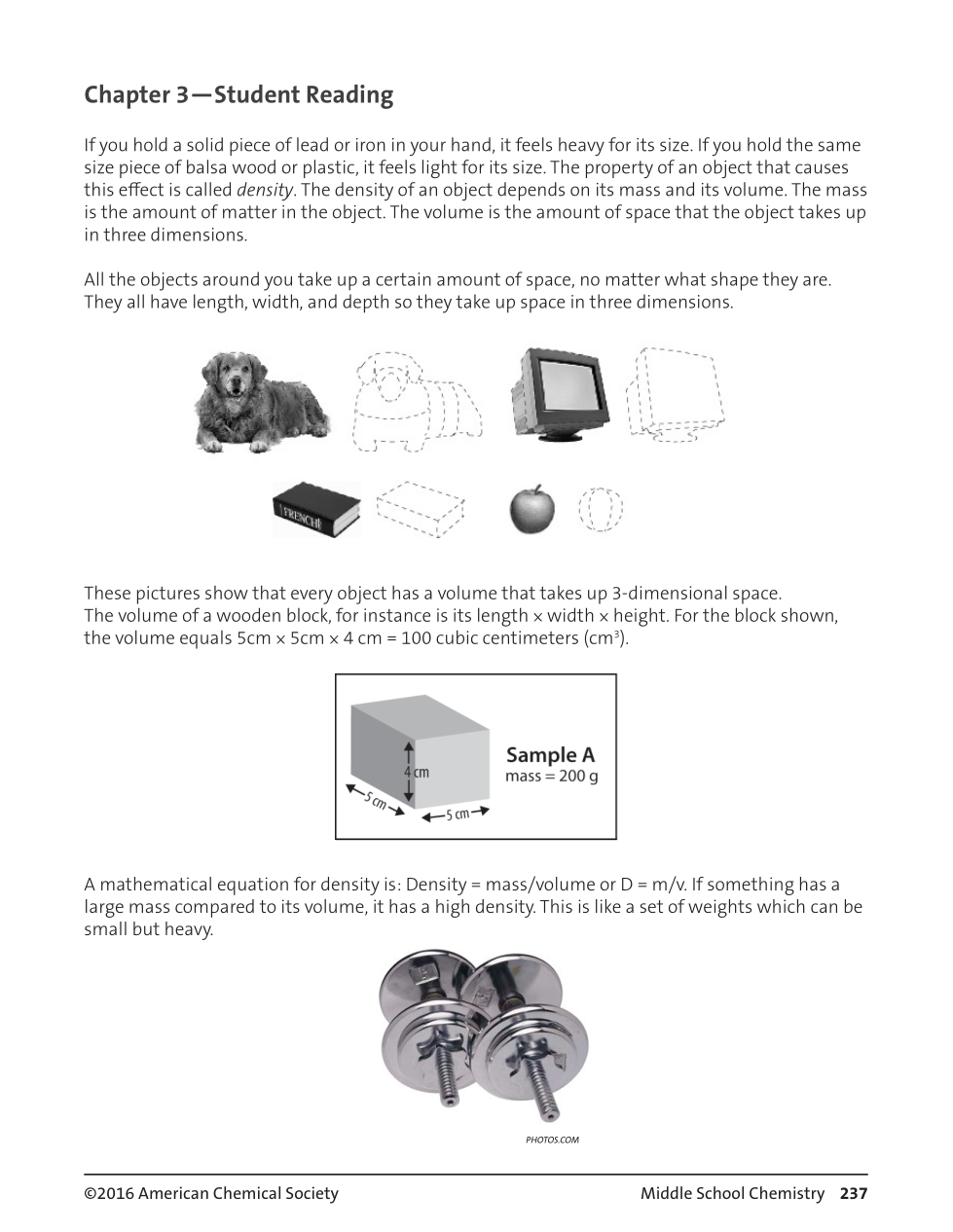
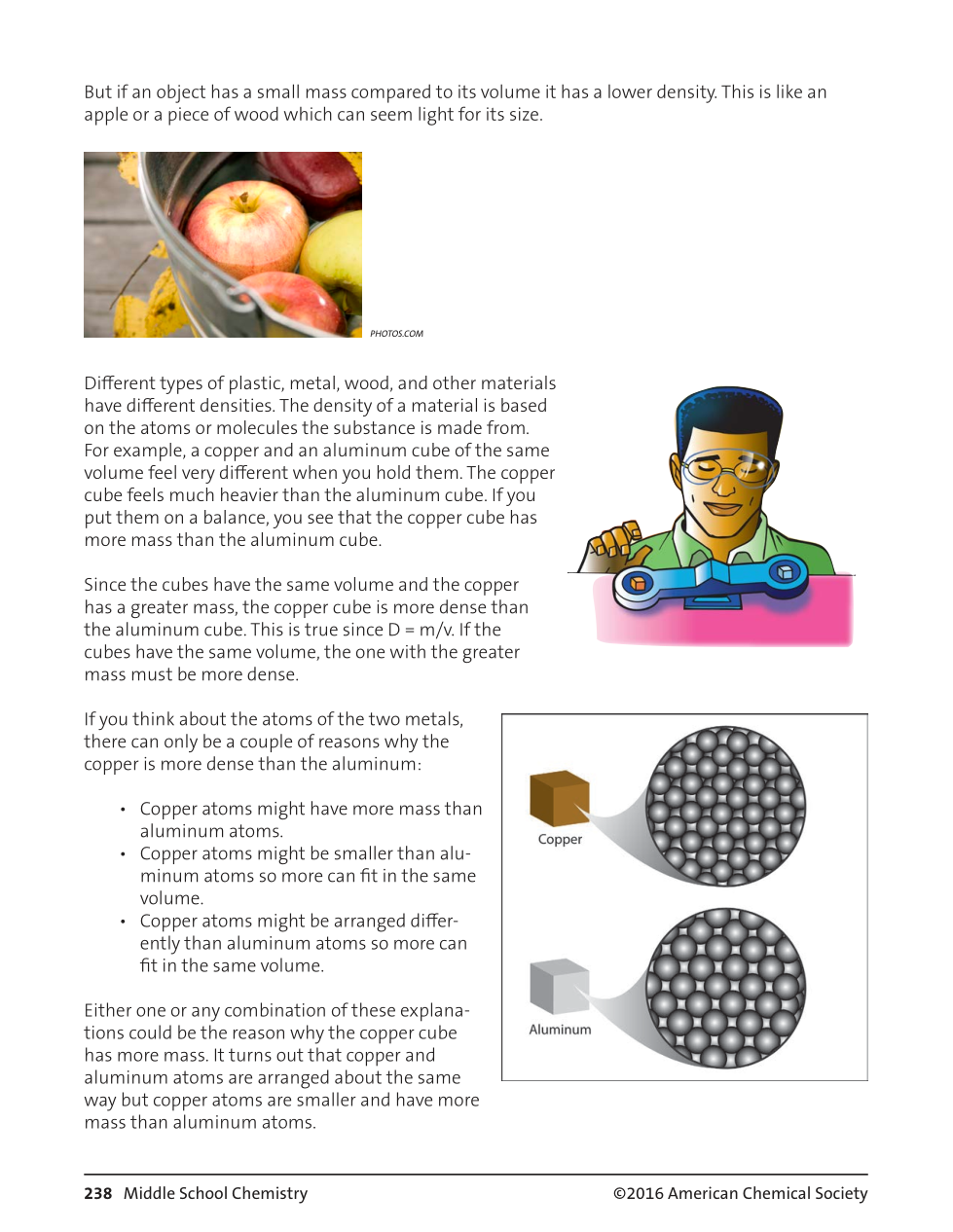
The initial volume of the water in a graduated cylinder is 60 mL. If you put an irregular shaped solid object in a graduated cylinder to find its volume, and the final volume of the water + the solid is 72 mL, that means the volume of the object is _______.