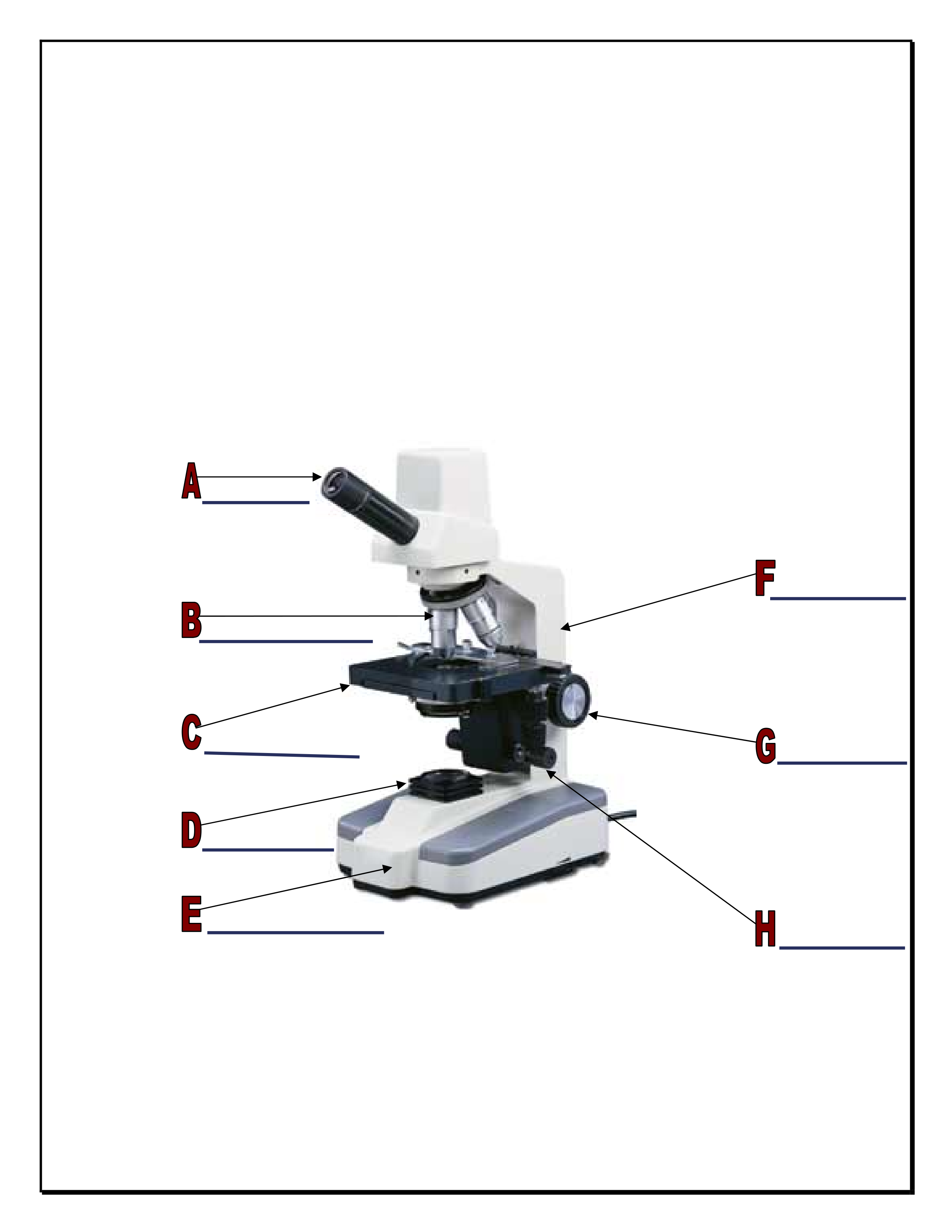
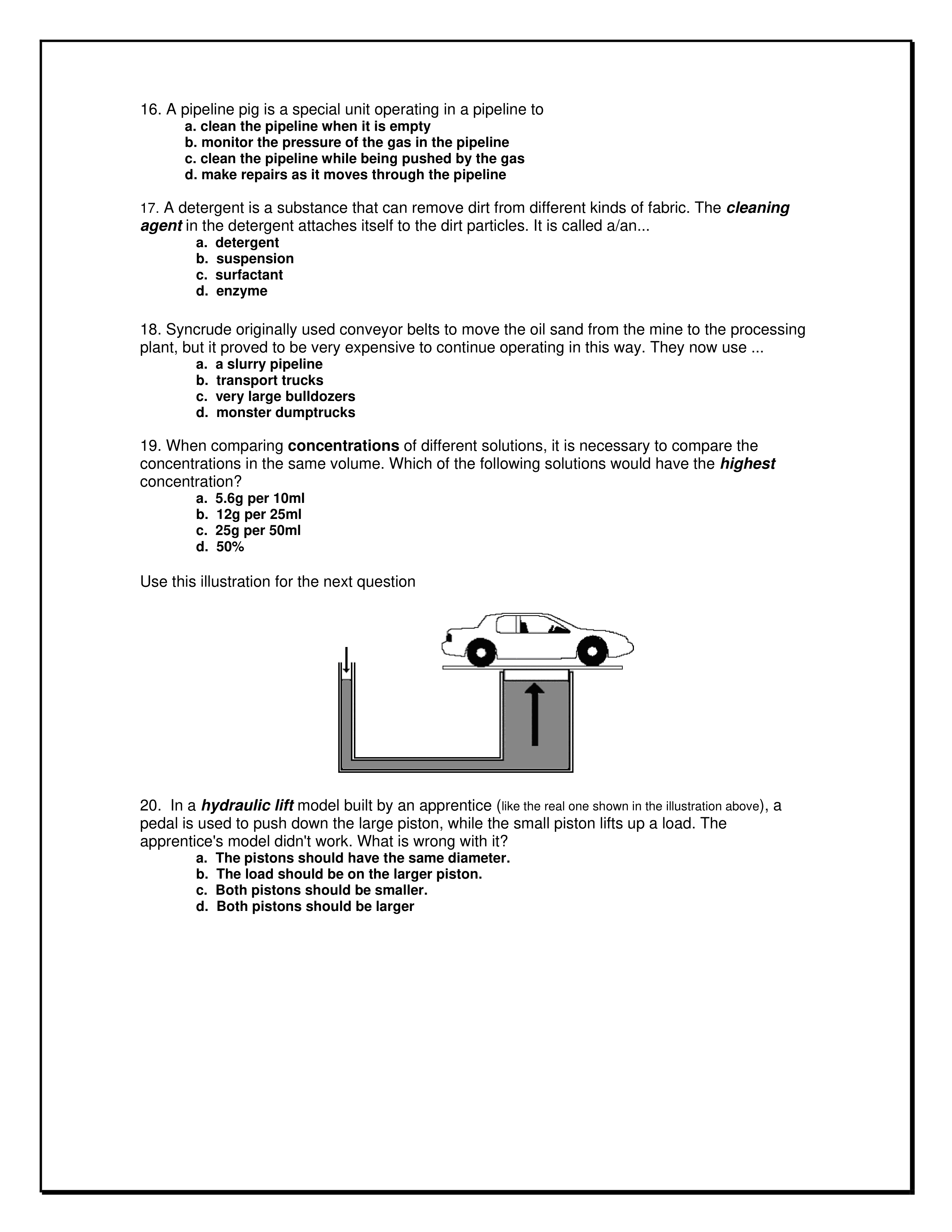
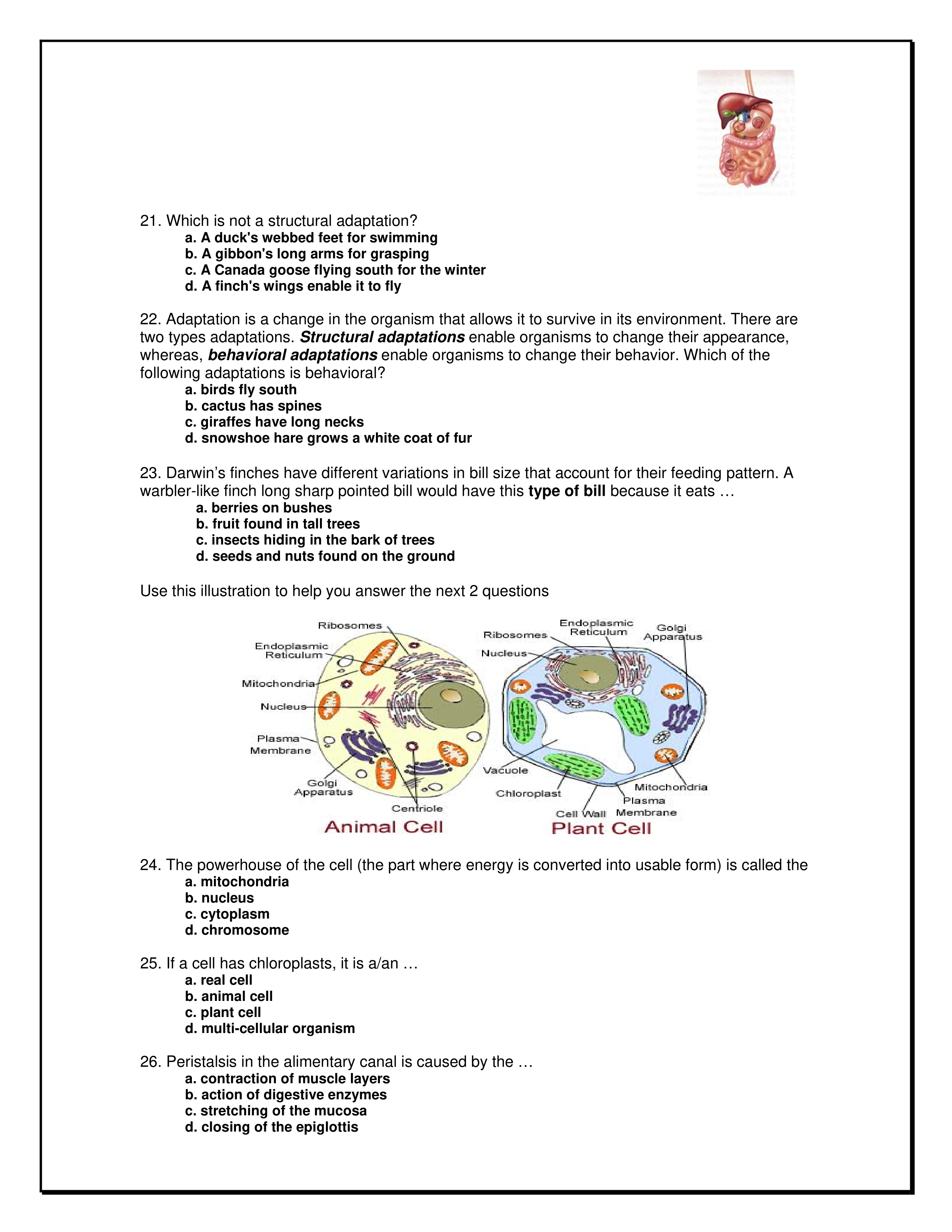
Use the following information to answer the question.
Carbon dioxide dissolved in water is acidic. Bromothymol blue is an acid/base indicator. A contaminated water sample is placed into a container containing some bromothymol blue. Another sample of pure water is placed into a similar container containing bromothymol blue. Both
containers were sealed and allowed to remain at room temperature, undisturbed, and in a darkened container for 24 h.
What would be a valid prediction for this experiment?