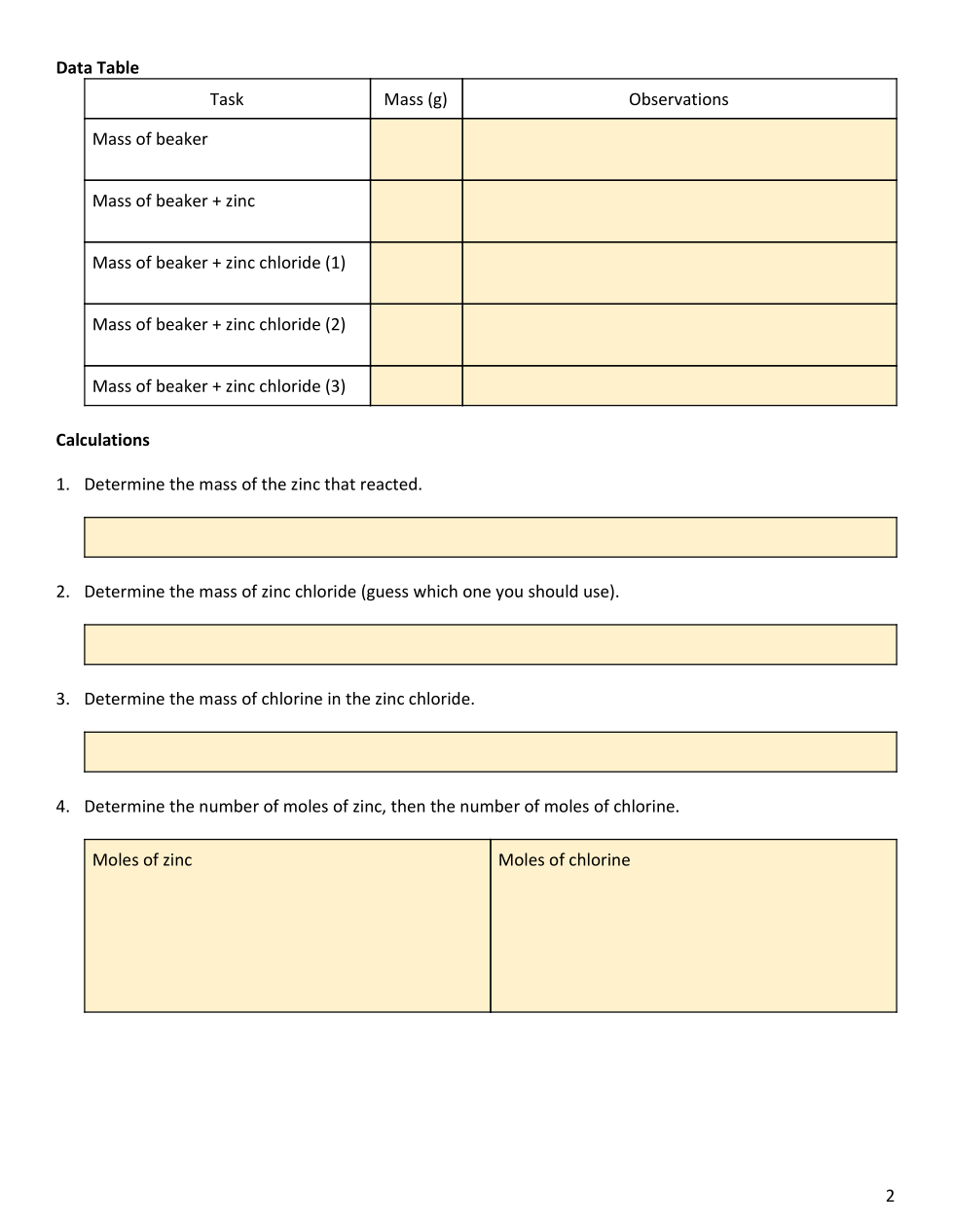
Empirical Formula Lab cloned 2/8/2021
star
star
star
star
star
Last updated about 5 years ago
9 questions
Note from the author:
The opening is from cK-12 online flexbook on chemistry chapter 7 section 2. The link is https://www.ck12.org/book/ck-12-chemistry-concepts-intermediate/section/7.2/