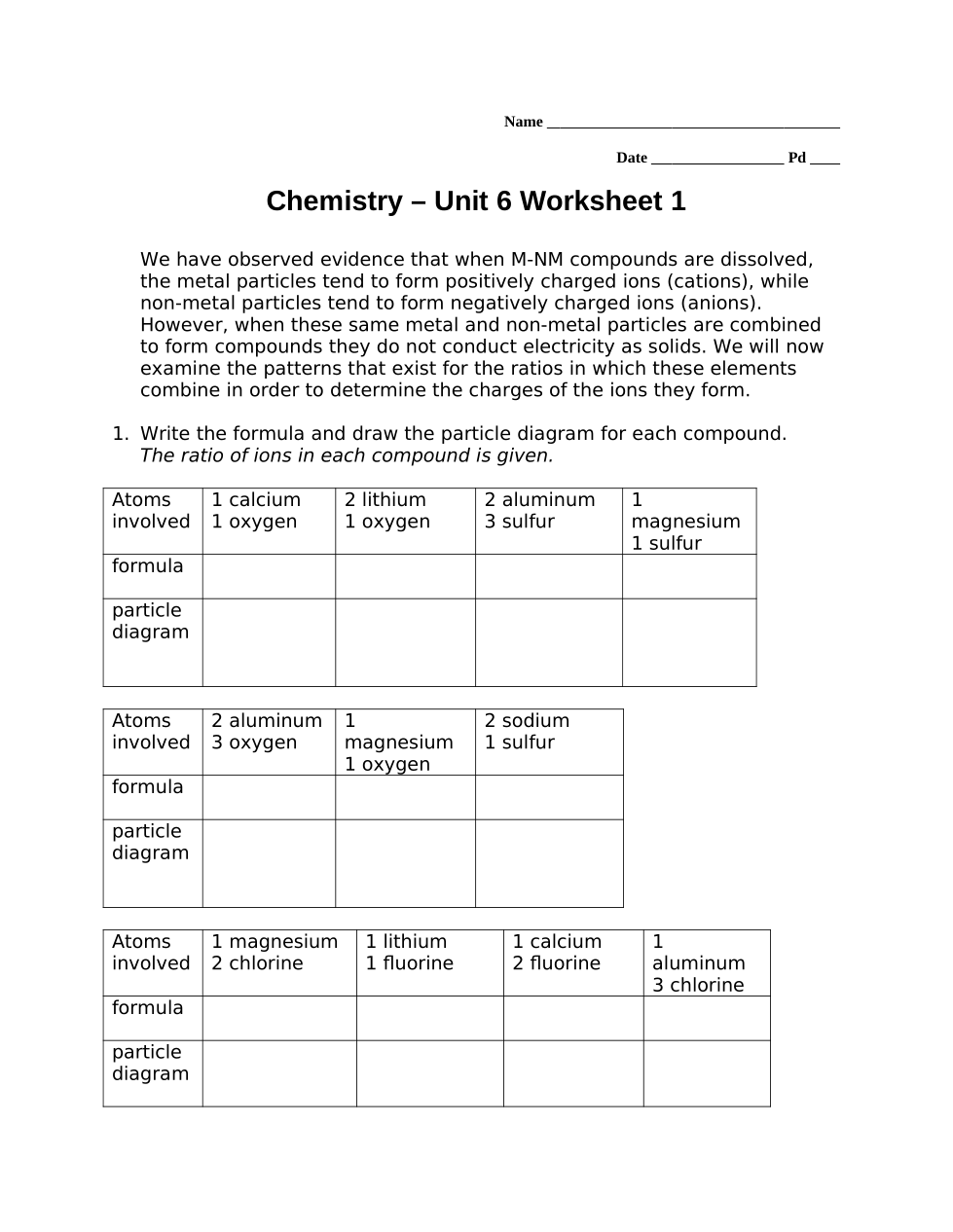
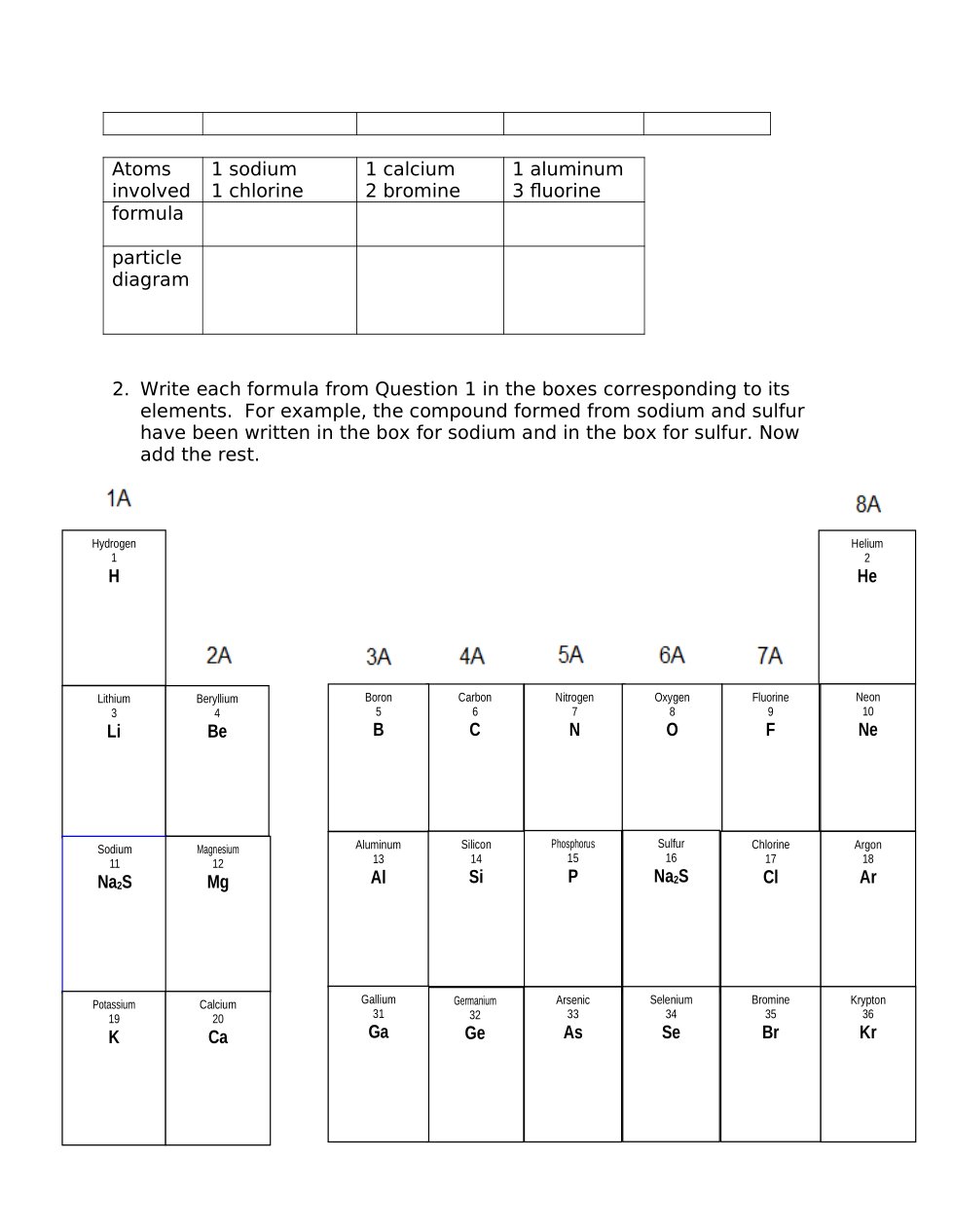
Make whatever generalizations you can about the charge of the ions formed by elements in columns 1A, 2A, 3A and 7A based on the ratio of atoms in each of the compounds they form. It might help to look at your particle diagrams in #1 and consider what charges the ions might have in order to result in neutral compounds.