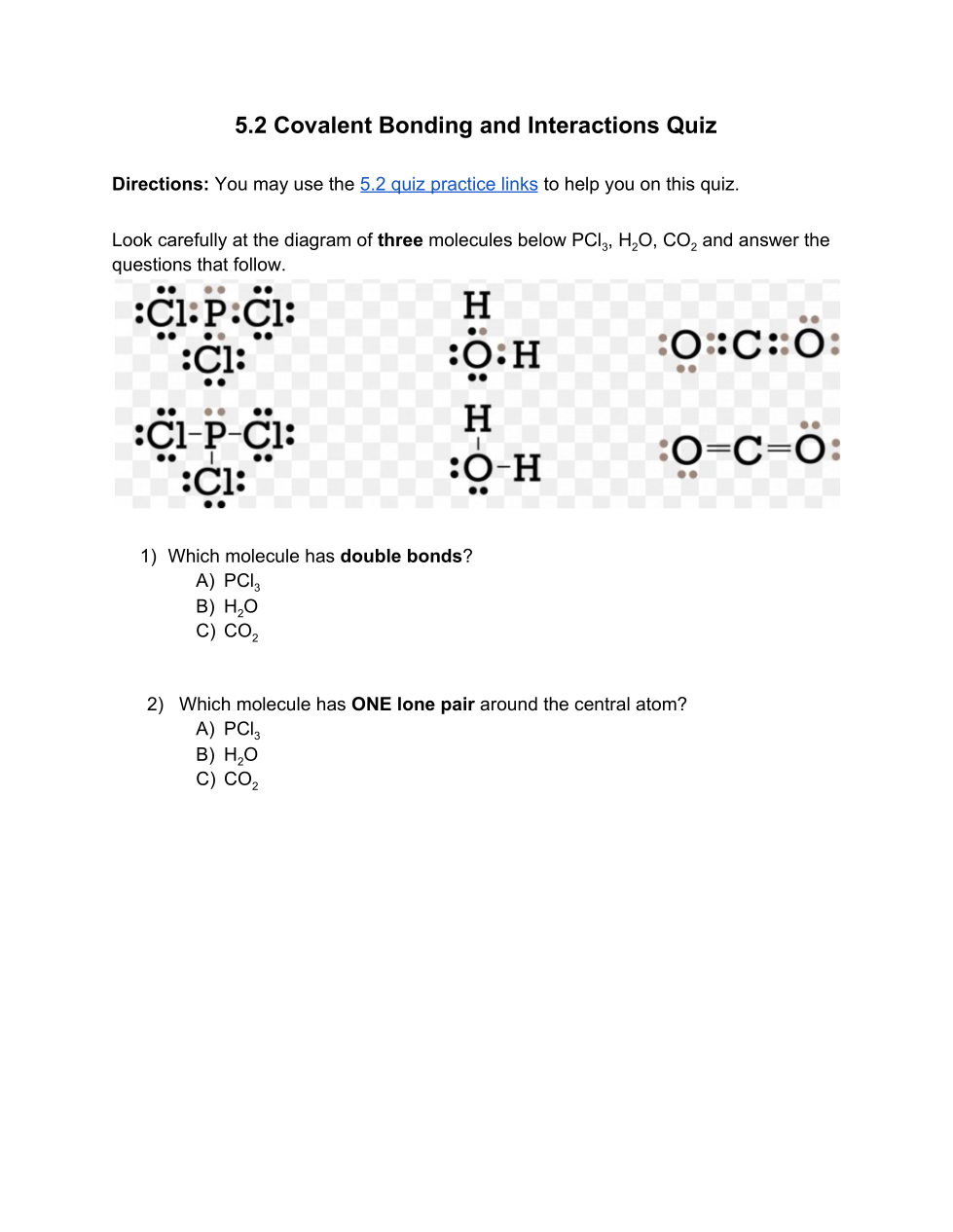
CLICK SHOW YOUR WORK TO FILL OUT THE TABLE FOR THIS QUESTION. AFTER YOU HAVE SHOWN YOUR WORK, THEN YOU CAN CHOOSE THE CORRECT ANSWER BELOW BASED ON YOUR MOLECULE. (Note: you must show the steps given for your molecule. Do not Google it...it is often wrong, incomplete, and doesn't follow the steps required)