7.4 Lemonade Lab
star
star
star
star
star
Last updated over 3 years ago
20 questions
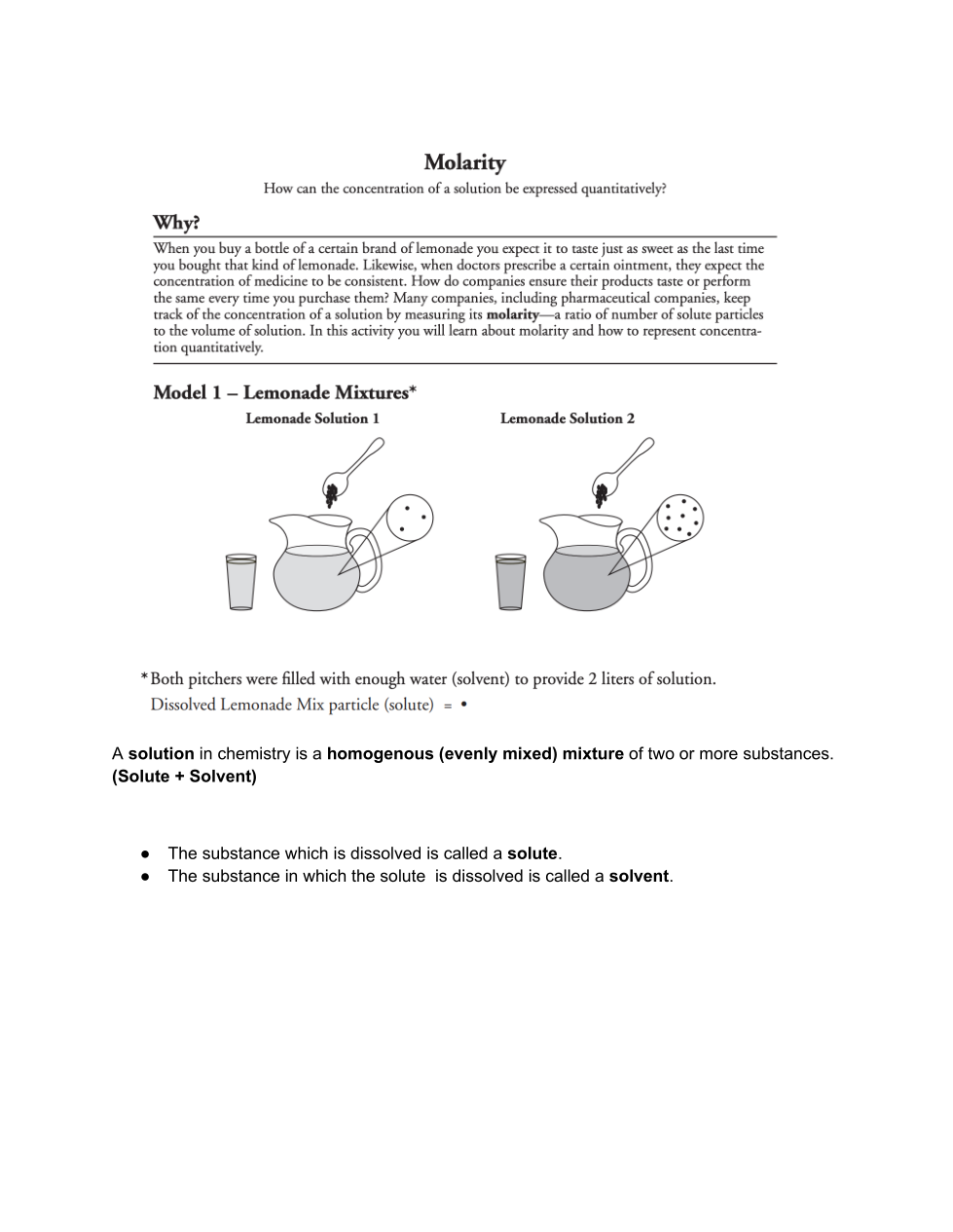







| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
Lemonade mix | arrow_right_alt | What is the solvent? |
Black Dot | arrow_right_alt | What is the solute? |
Water | arrow_right_alt | What is a dissolved lemonade mix particle represented by? |