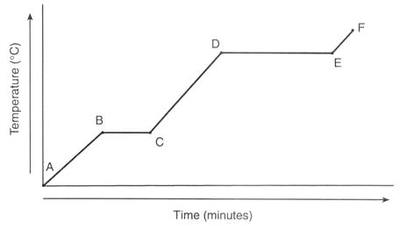
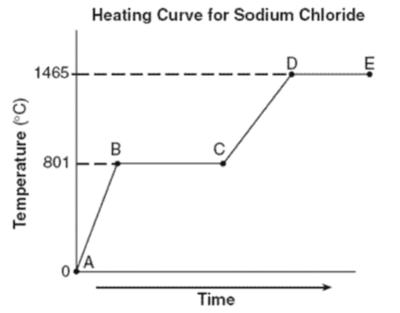
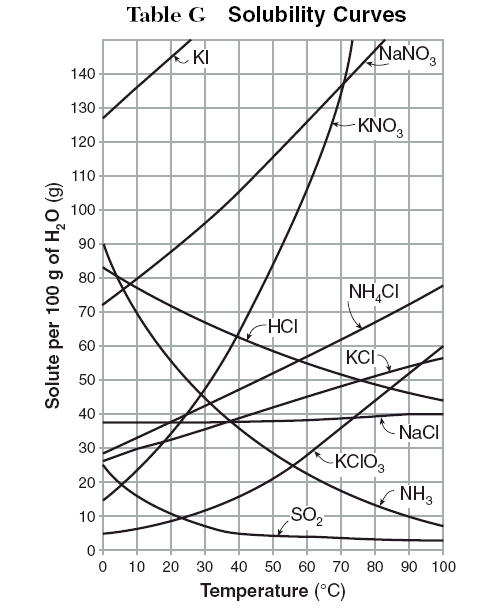
Unit 6 Physical Science
star
star
star
star
star
Last updated over 4 years ago
39 questions
1
1
2
2
1
1
3
1
1
1
1
1
1
1
1
1
1
1
1
2
2
1
1
1
5
1
1
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
sublimation | arrow_right_alt | gas to liquid |
condensation | arrow_right_alt | liquid to solid |
melting | arrow_right_alt | solid to gas |
deposition | arrow_right_alt | liquid to gas |
freezing | arrow_right_alt | solid to liquid |
vaporization | arrow_right_alt | gas to solid |
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
mud puddle | arrow_right_alt | colloid |
salt water | arrow_right_alt | solution |
carbon dioxide (CO2) | arrow_right_alt | suspension |
gold (Au) | arrow_right_alt | element |
milk | arrow_right_alt | compound |