NGSS Chemistry NCSD Unit 1 Test - A (master)
star
star
star
star
star
Last updated over 3 years ago
17 questions
What can you use on the quiz?
- Your notes
- Your brain
- Not other people or the internet
You need to make sure you are answering questions in your own words. GoFormative will check your quiz against other students and the internet.
Standards
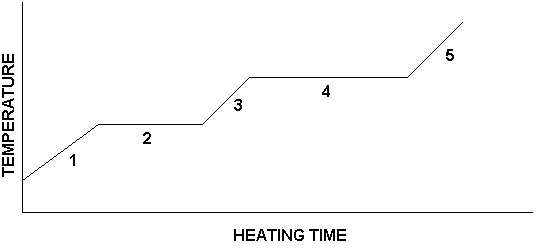
- I can model and explain the motion and relative position of particles in each phase and how that is associated with energy.
- I can model and explain what occurs during a phase change in terms of energy, motion, and relative position of particles.
- I can identify and describe the inverse and proportional mathematical relationships between temperature, pressure, and volume of gases as well as describe what will happen to the particles of a gas when temperature, pressure, or volume is changed.
- I can develop and use models to describe phenomena.
2
3
3
3
1
2
1
1
1
1
4