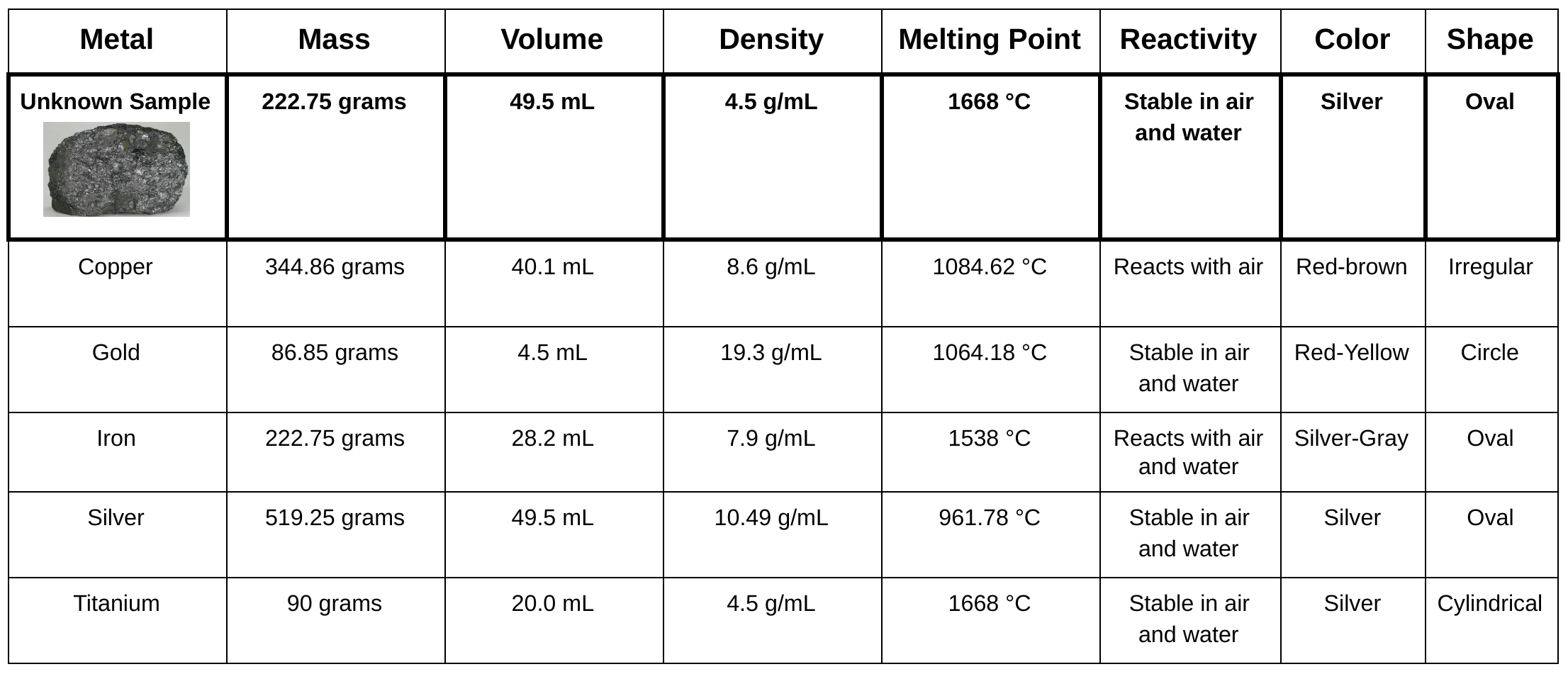
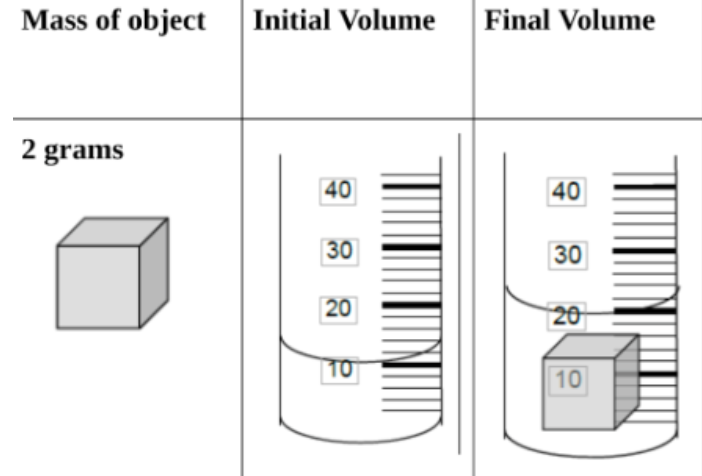
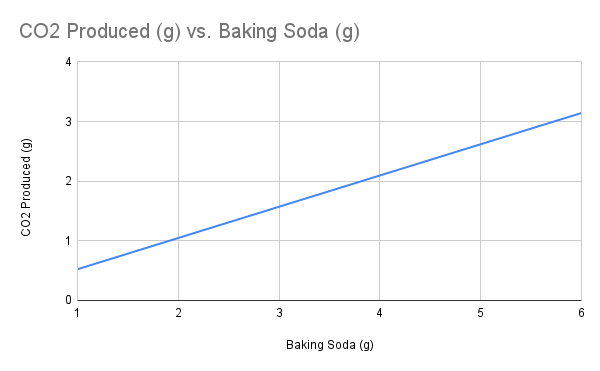
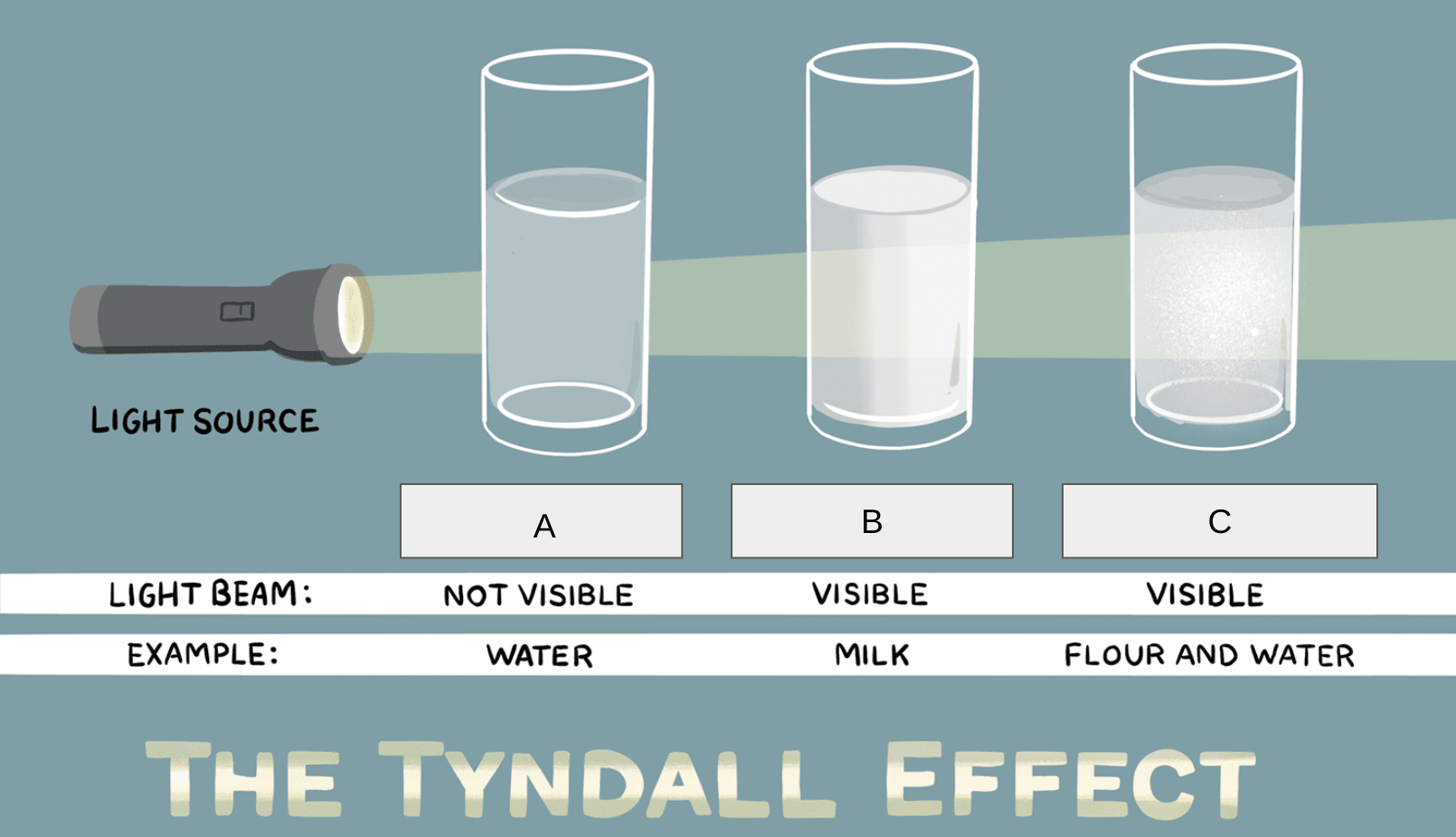
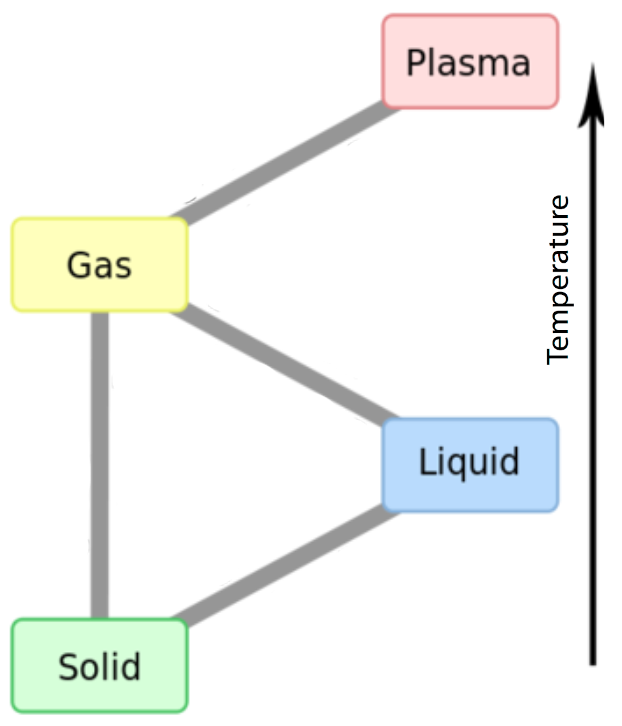
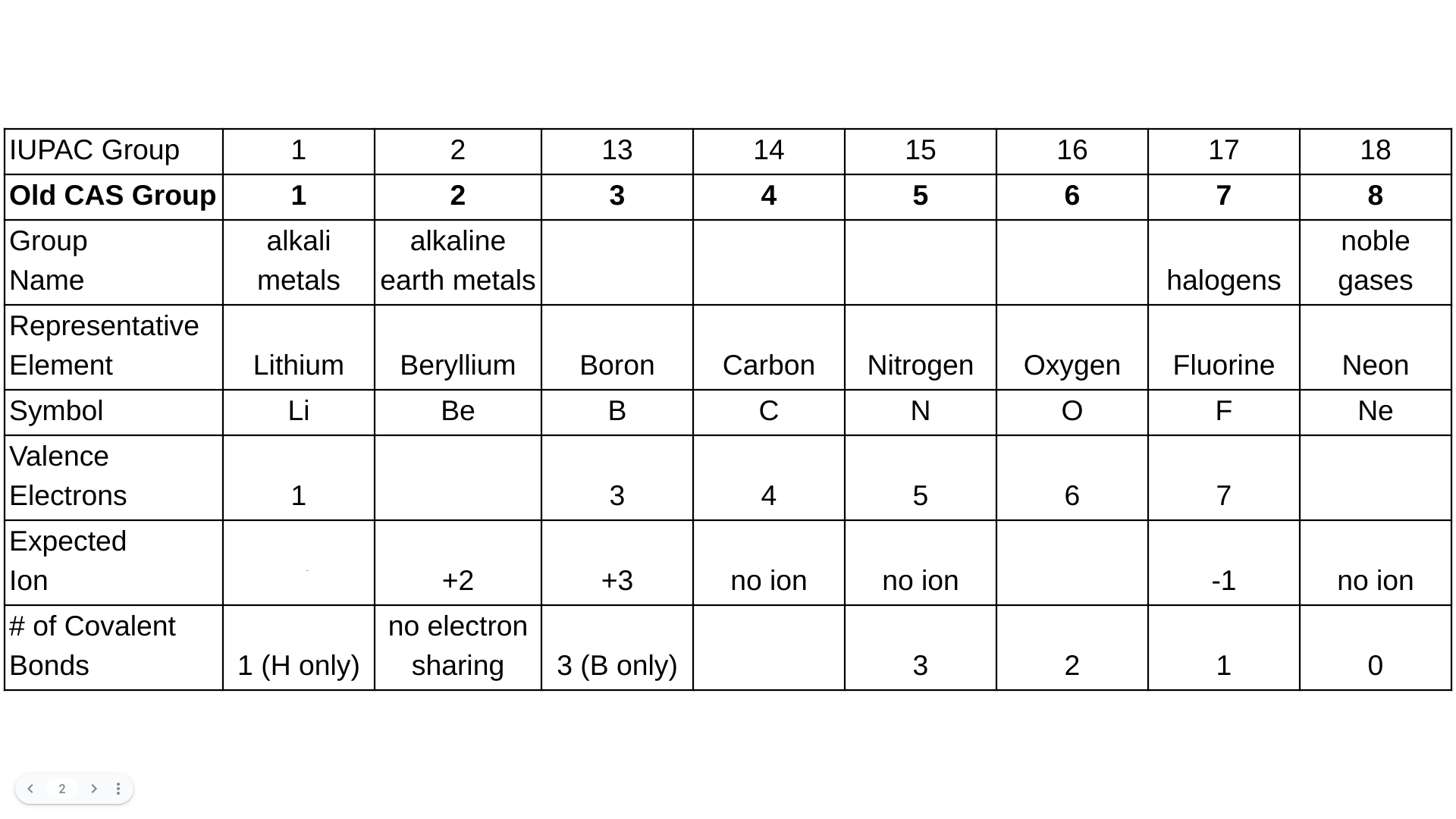
Unit 1: The Structure of Matter Assessment
star
star
star
star
star
Last updated over 2 years ago
123 questions
Heine Question Bank
4
2
2
2
1
1
3
3
4
1
1
8
4
1
1
1
1
1
1
1
1
1
5
1
1
1
1
1
1
1
1
1
6
1
1
3
1
1
1
1
1
1
3
2
2
2
2
5
1
1
1
6
Lynch Question Bank
1
Required
1
2
Required
1
Required
1
Required
3
Required
1
Required
7
Required
0.5
Required
0.25
1
1
1
1
1
1
1
1
1
1
1
1
5
2
2
2
2
2
4
Lock Question Bank
1
1
1
1
1
7
1
1