2.4 Build an Atom Phet Simulation
star
star
star
star
star
Last updated over 3 years ago
21 questions
1







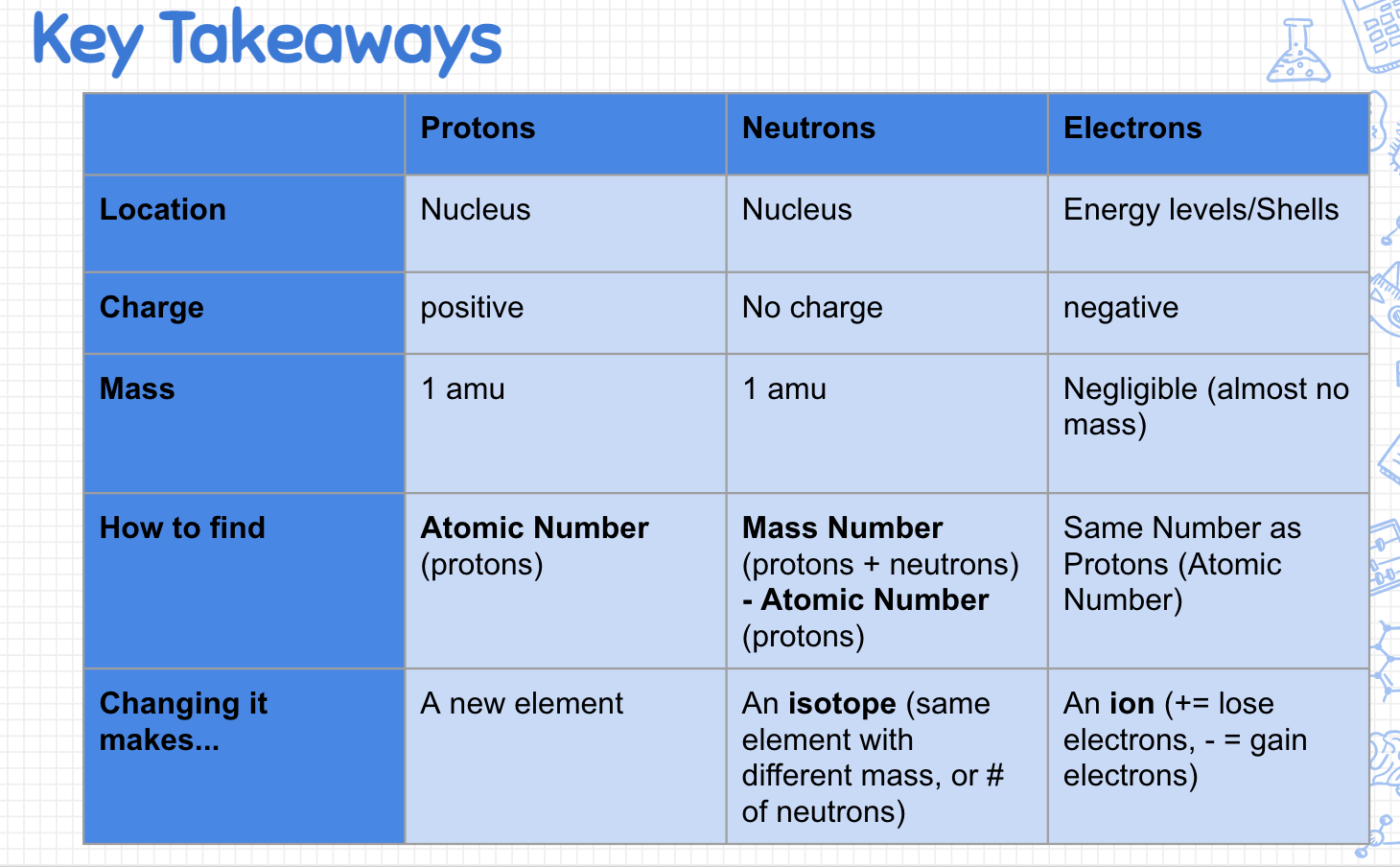
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
Add/Remove Protons | arrow_right_alt | A new element forms |
Add/Remove Neutrons | arrow_right_alt | Same element with a different mass |
Add/Remove Electrons | arrow_right_alt | Forms a charge/ion of the same element |