4.1 - Ions
star
star
star
star
star
Last updated over 4 years ago
24 questions
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
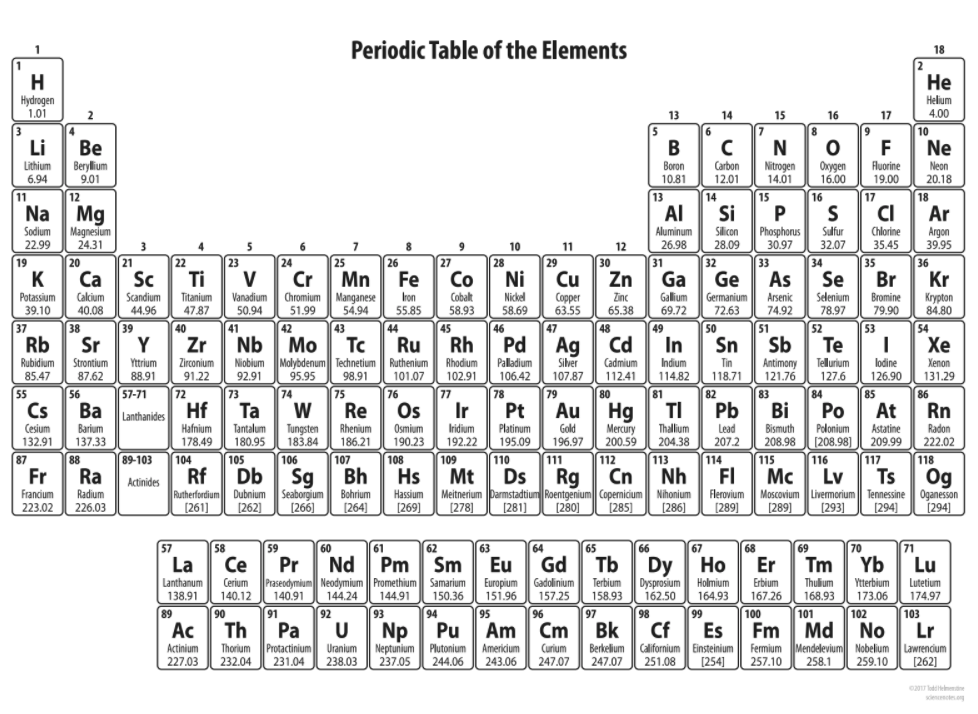
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
cation | arrow_right_alt | |
79 | arrow_right_alt | |
80 | arrow_right_alt | |
19 | arrow_right_alt | |
36 | arrow_right_alt | |
22 | arrow_right_alt | |
10 | arrow_right_alt | |
2+ | arrow_right_alt | |
anion | arrow_right_alt |