Metallic Bonding 21-22
star
star
star
star
star
Last updated about 4 years ago
5 questions
1
1
1
1
6
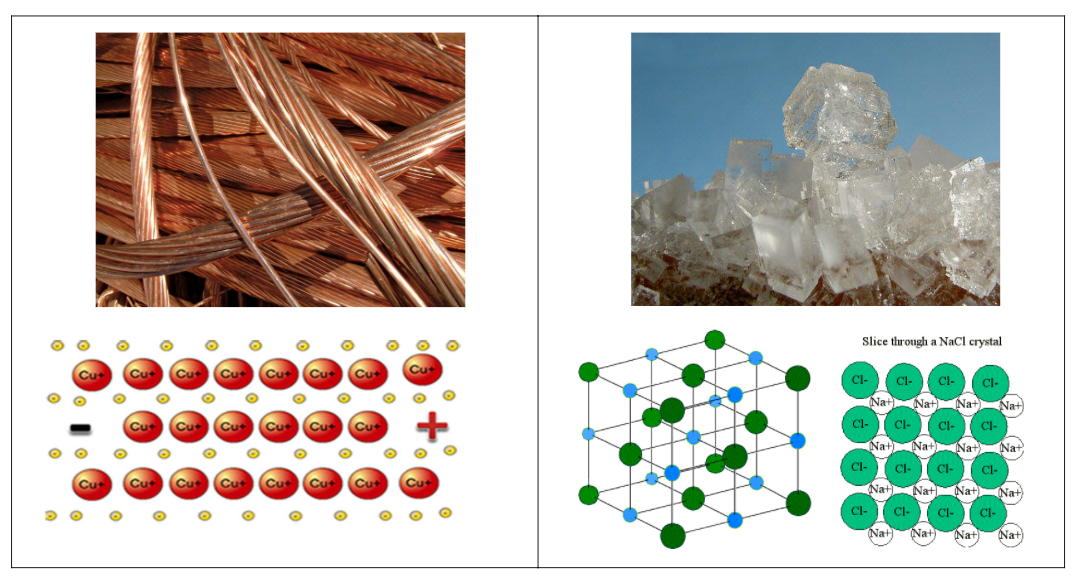
Use the above image of copper metal and dodium chloride in your evidence to justify why metals are able to be drawn into a thin wire and not ionic salts. Be sure to give detailed informationby both using and underlining or bolding the following 4 terms in your explanation: cation, anion, sea of electrons, and chemical bond.
Use the above image of copper metal and dodium chloride in your evidence to justify why metals are able to be drawn into a thin wire and not ionic salts. Be sure to give detailed informationby both using and underlining or bolding the following 4 terms in your explanation: cation, anion, sea of electrons, and chemical bond.