126 Chemical Formulas-Notes and Models
star
star
star
star
star
Last updated over 3 years ago
17 questions
Note from the author:
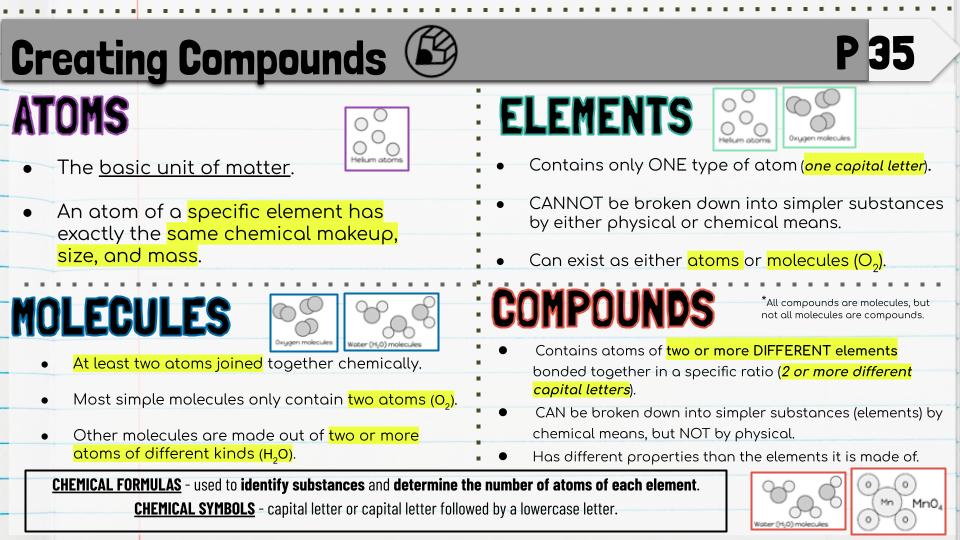
TEKS: I CAN recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts (8.5D)
Essential Question: How can we determine the number of atoms in chemical formulas containing subscripts, coefficients, and parentheses? We will discuss chemical formulas, what they represent, and how to count the atoms represented by them.
Pay attention to:
- Important vocubulary
- How atoms combine
- Steps to count atoms
TEKS: I CAN recognize that chemical formulas are used to identify substances and determine the number of atoms of each element in chemical formulas containing subscripts (8.5D)
Set up journal page 35.
Essential Question: How can we determine the number of atoms in chemical formulas containing subscripts, coefficients, and parentheses?
We will discuss chemical formulas, what they represent, and how to count the atoms represented by them.
Pay attention to:
- Important vocubulary
- How atoms combine
- Steps to count atoms
Be sure to write examples of how to count atoms in a chemical formula.
0
0
0
0
0
0