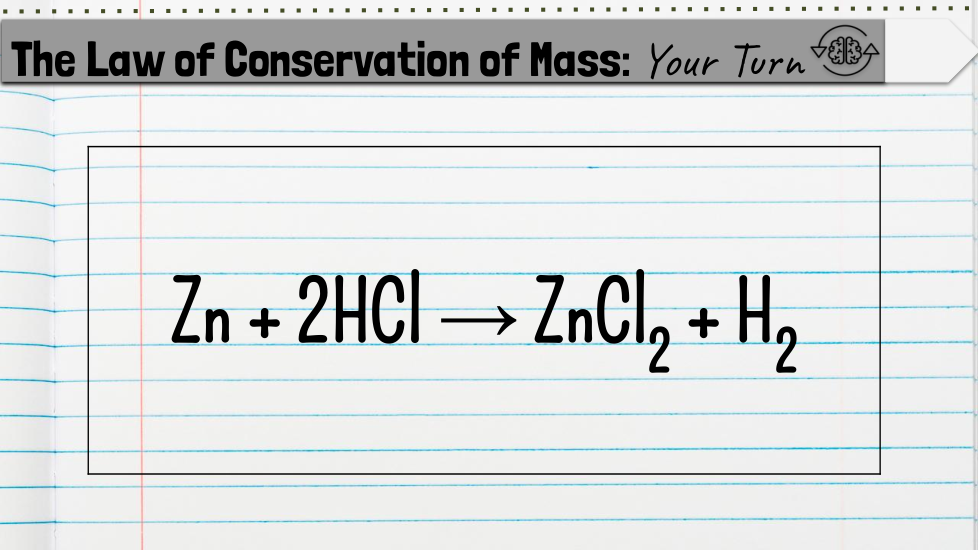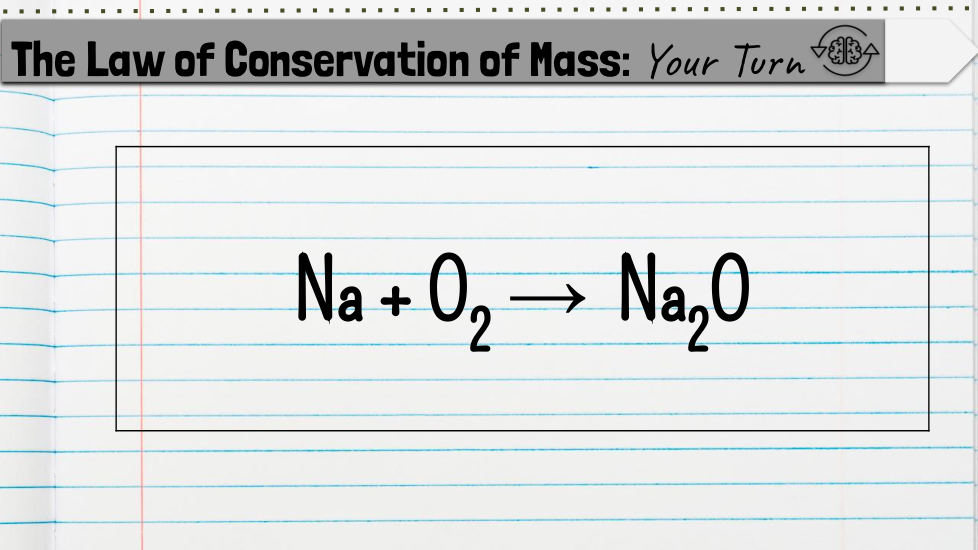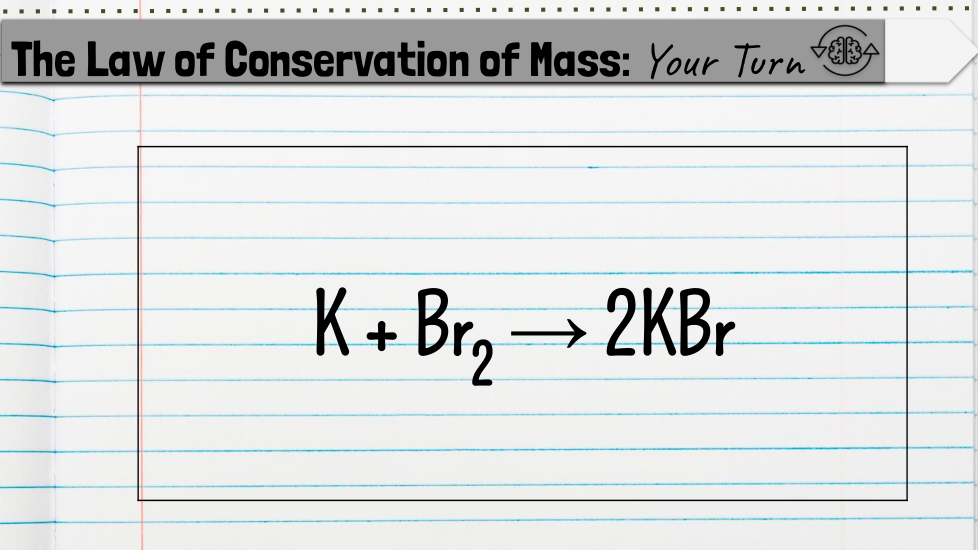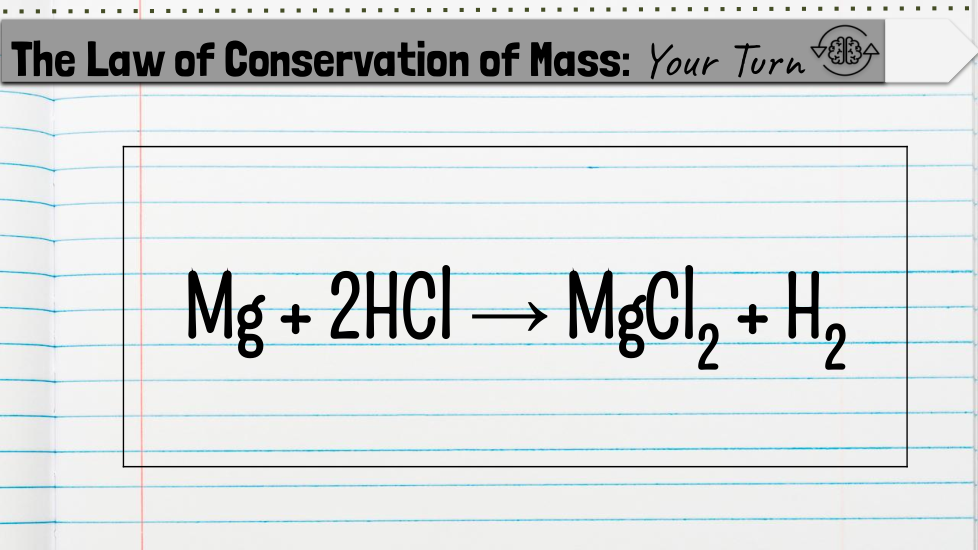134 Chemical Equations-Mastery Check
star
star
star
star
star
Last updated over 3 years ago
4 questions
Note from the author:
Students will count the atoms of a chemical equation to determine whether it is balanced or unbalanced based on the law of conservation of mass.