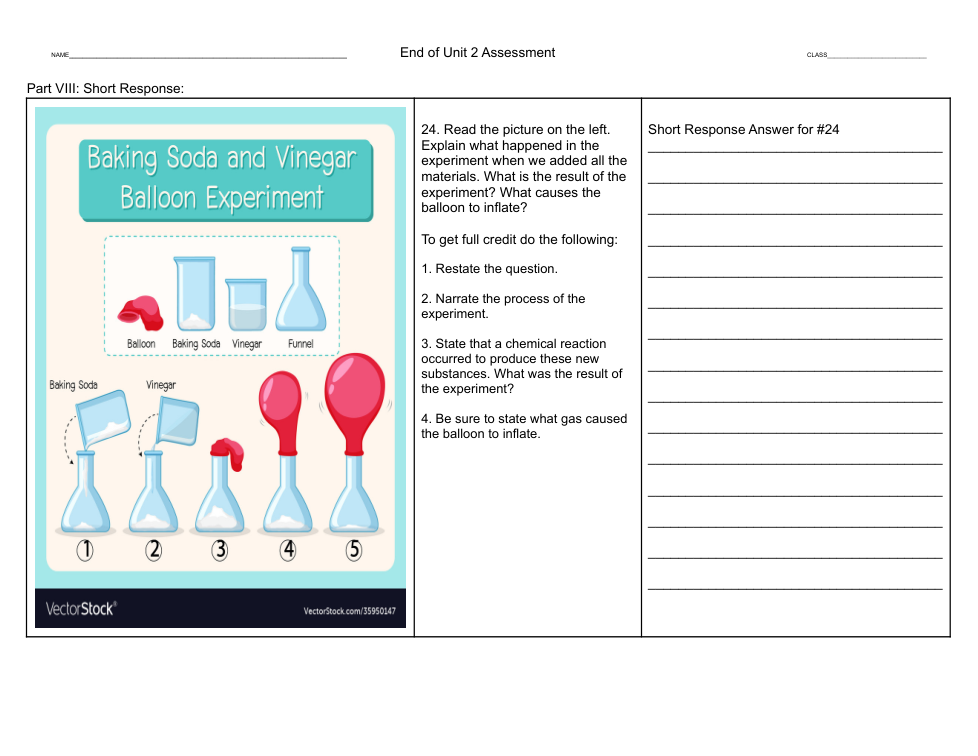
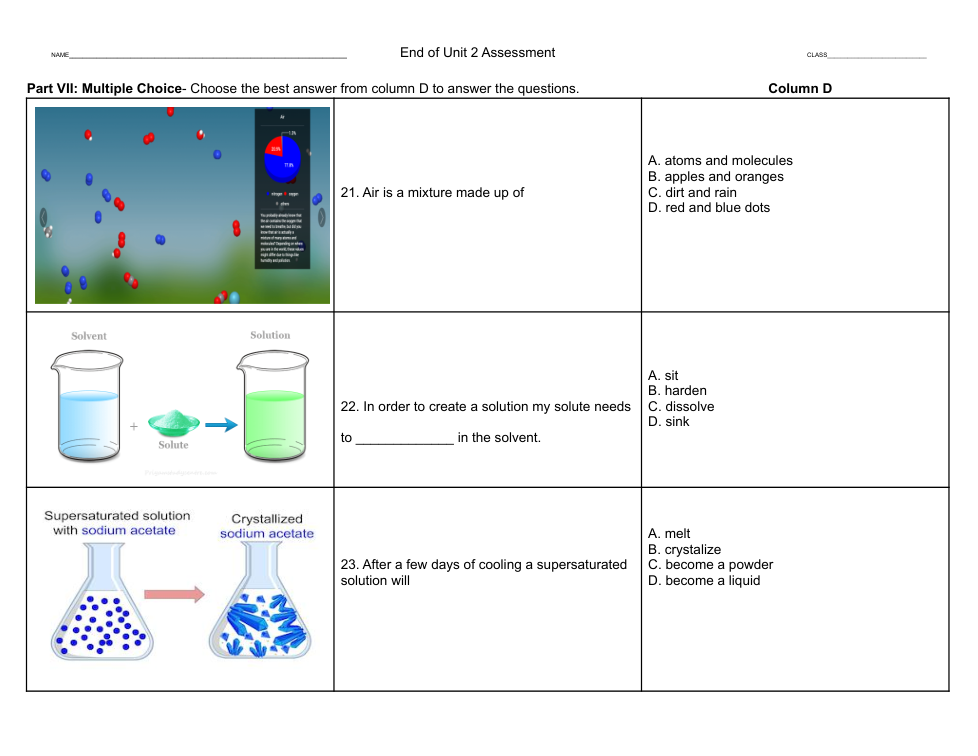
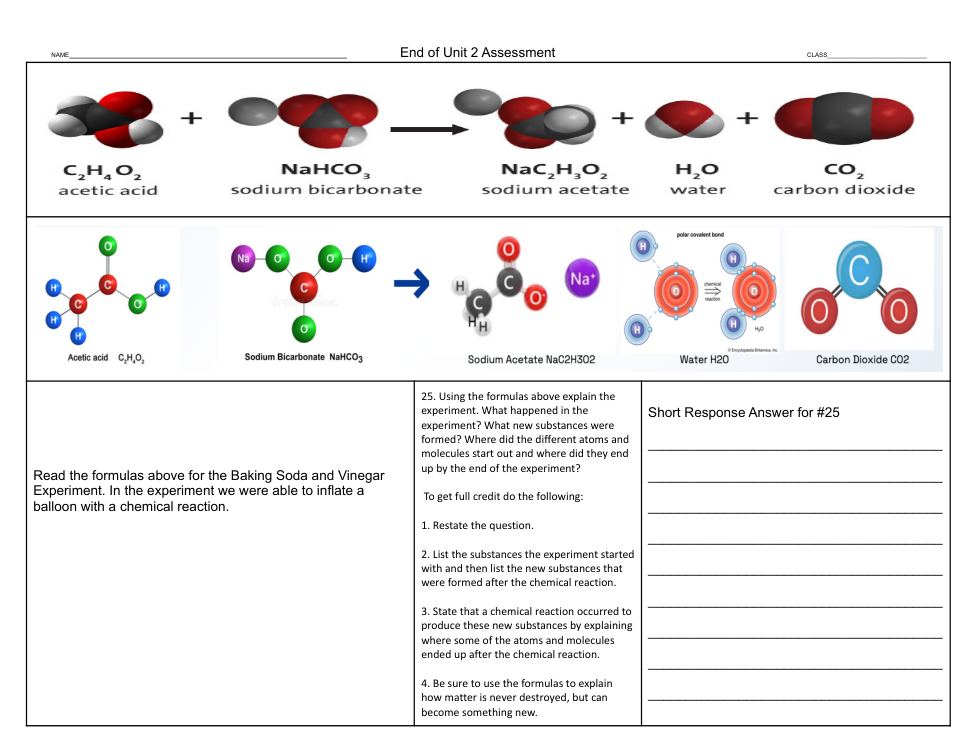
Using the formulas above explain the experiment. What happened in the experiment? What new substances were formed? Where did the different atoms and molecules start out and where did they end up by the end of the experiment?
To get full credit do the following:
2. List the substances the experiment started with and then list the new substances that were formed after the chemical reaction.
3. State that a chemical reaction occurred to produce these new substances by explaining where some of the atoms and molecules ended up after the chemical reaction.
4. Be sure to use the formulas to explain how matter is never destroyed, but can become something new.