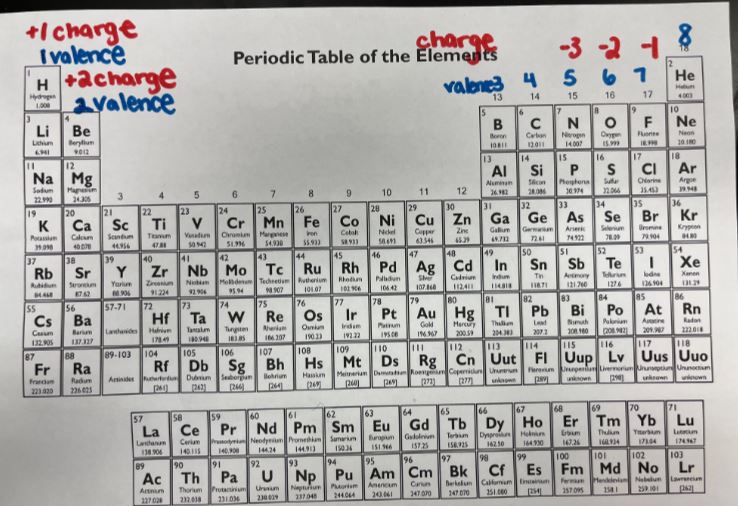
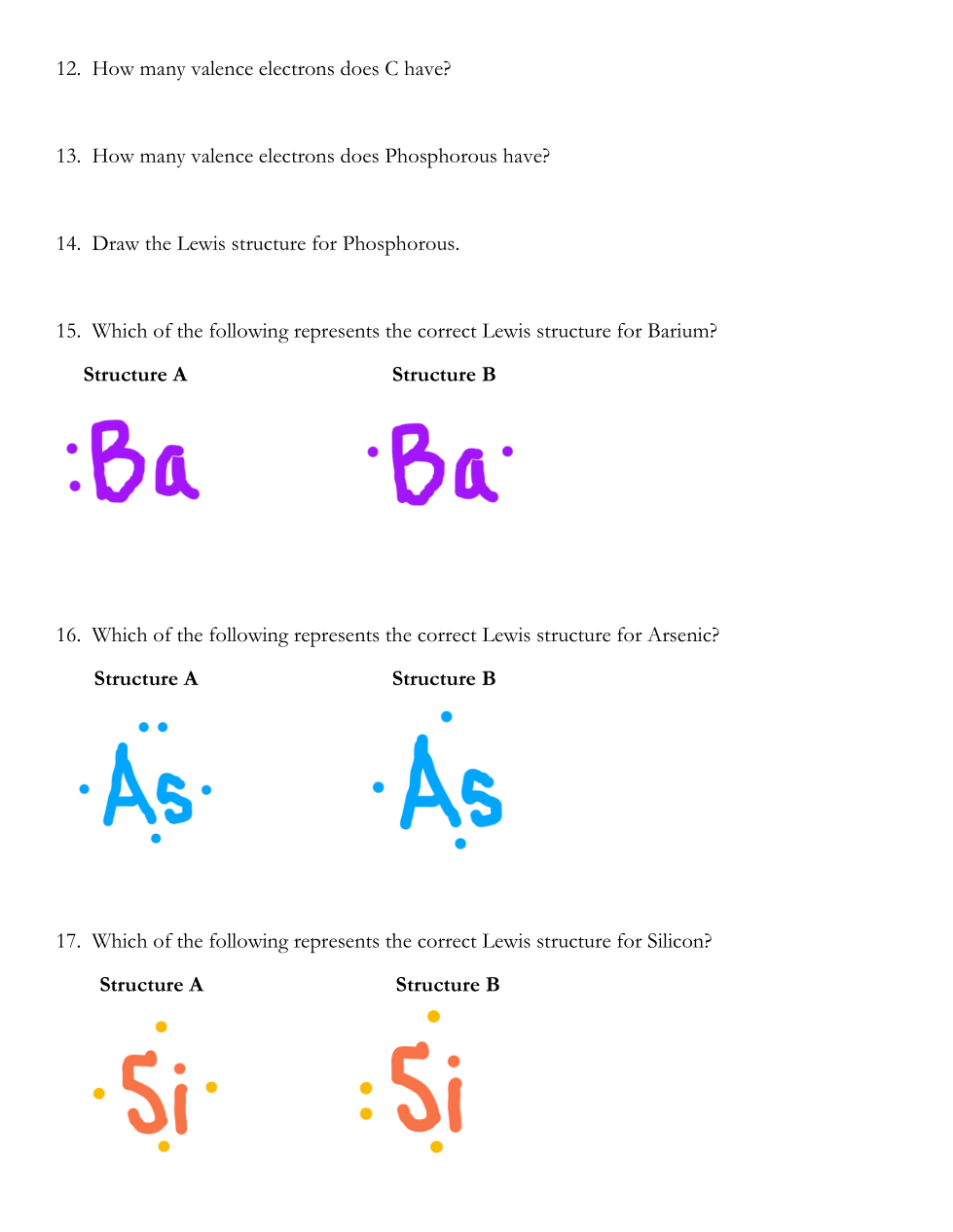
Review Notes For Questions 1-11:
Metals: Become positive ions, and give away/lose electrons
Nonmetals: Become negative ions, and gain or accept electrons
Ionic Compounds: made up of a positive (metal) and a negative nonmetal, in which the metal gives electrons to the nonmetal