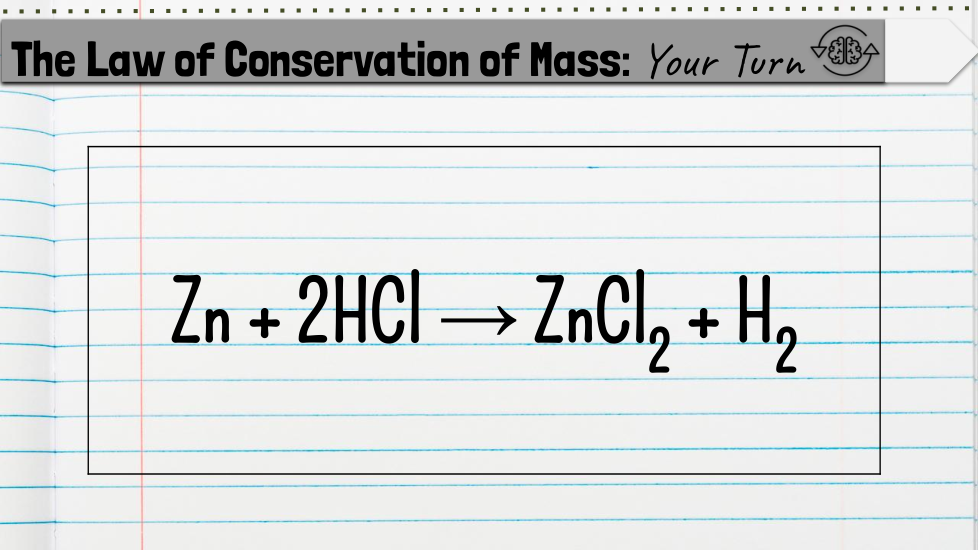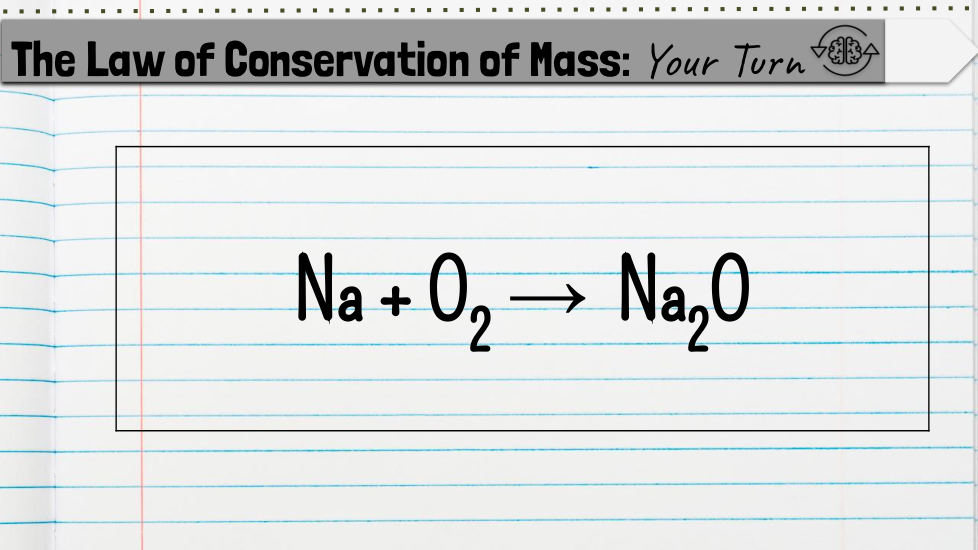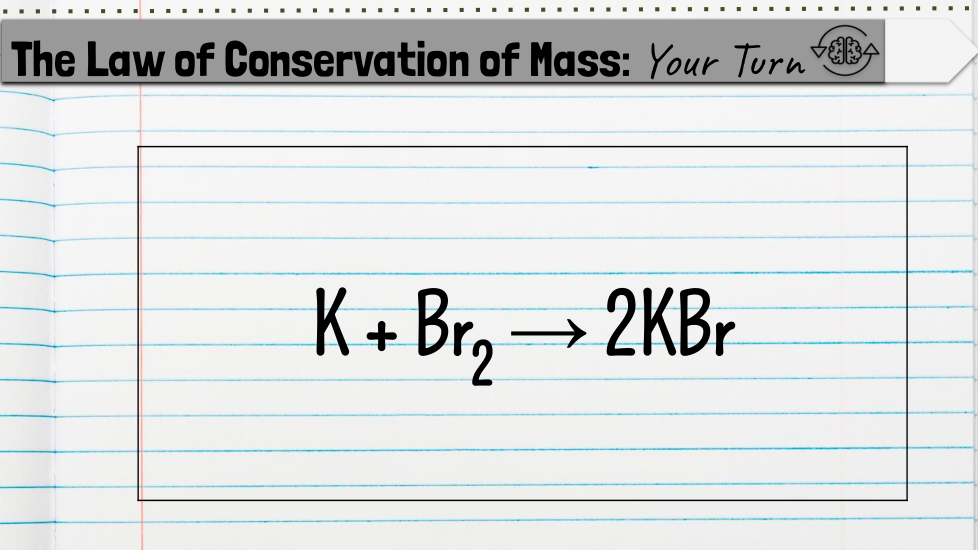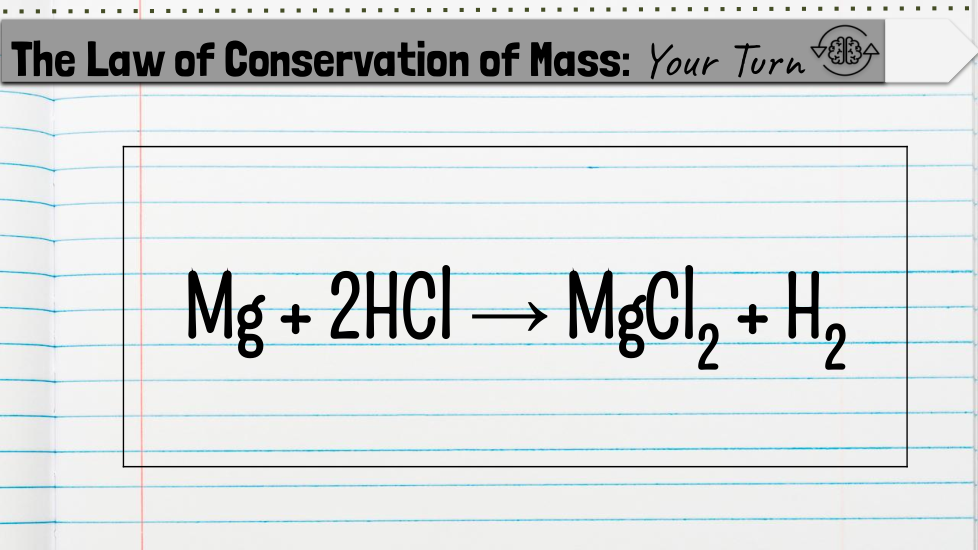Engage: Conservation of Mass with Alka Seltzer and Water Reaction
star
star
star
star
star
Last updated over 3 years ago
16 questions
Watch the video of the chemical reaction between alka seltzer and water. This reaction will prove our new concept of the Law of Conservation of Mass.
Conservation of Mass Demonstration
Chemical Formulas