SDAIE: DE_LivingEarth_2.1_Energy for Life
star
star
star
star
star
Last updated over 2 years ago
39 questions
Engage
Required
2
Required
2
Required
1
Required
1
Required
1
Required
2
Required
2
Required
7
Required
1
Required
1
Required
1
Explore #1: What Is Energy and How Does It Contribute to Maintaining Life on Earth?
Required
6
Required
2
Required
1
Required
1
Required
1
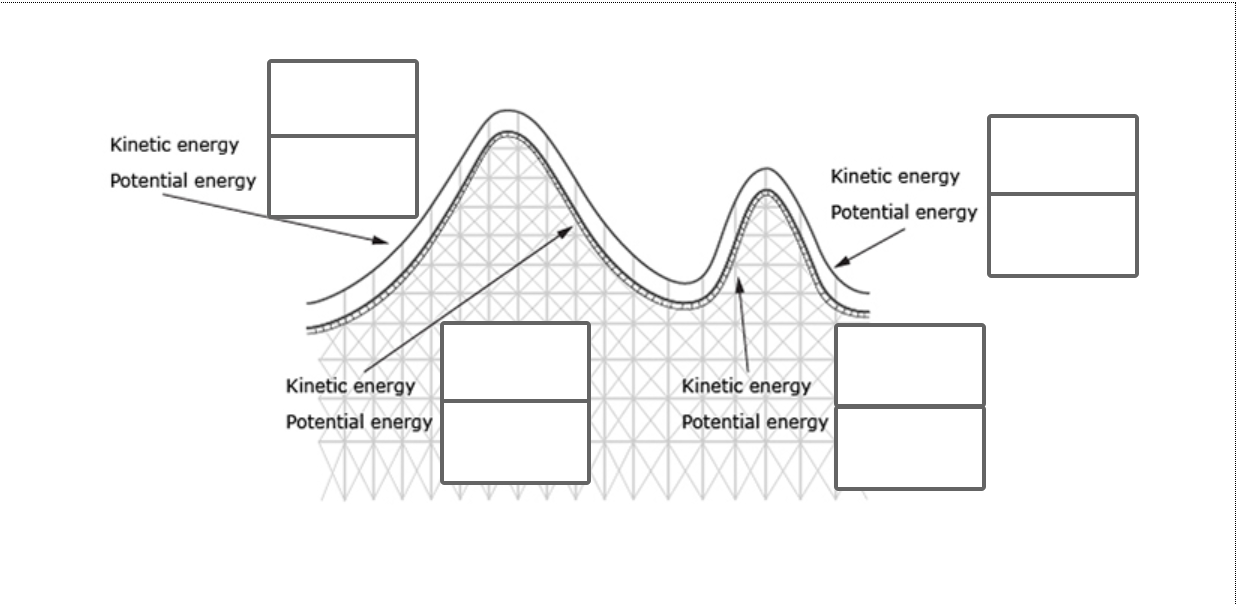
Explore #2: What are Kinetic and Potential Energy?
Required
3
Required
2
Required
1
Required
1
Required
1
Required
1
Required
1
Explore #3 "What are the laws of thermodynamics"?
Explain #4: "What Is the Difference between Endergonic and Exergonic Reactions?"
Required
2
Explore #5: "What Is the Difference between Oxidation and Reduction Reactions?"
Required
1
Required
4
Required
5