DE_Chem_3.2_IonicBonding
star
star
star
star
star
Last updated over 2 years ago
22 questions
Note from the author:
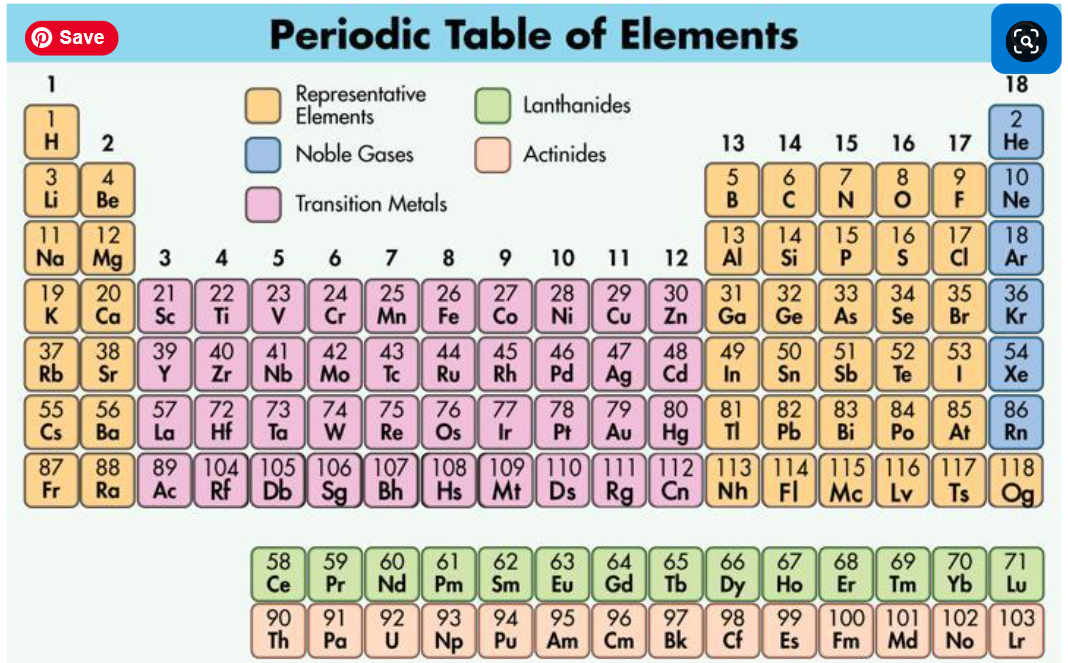
Refer to Discovery Education's chemistry text: Unit 3.2 Ionic Bonding to answer the following questions. Additional questions may be added so check your work before turning in and make sure everything has been responded to.
Refer to Discovery Education's chemistry text: Unit 3.2 Ionic Bonding to answer the following questions. Additional questions may be added so check your work before turning in and make sure everything has been responded to.
Enagage: Deconstructing Salts
Required
1
Required
1
Required
2
Required
8
Explore 1
Required
1
Required
3
Required
2
Required
1
Explore 2
Required
3
Required
1
Required
1
Required
2
Required
1
Check for Understanding (Quiz)
1
1
1
1
1