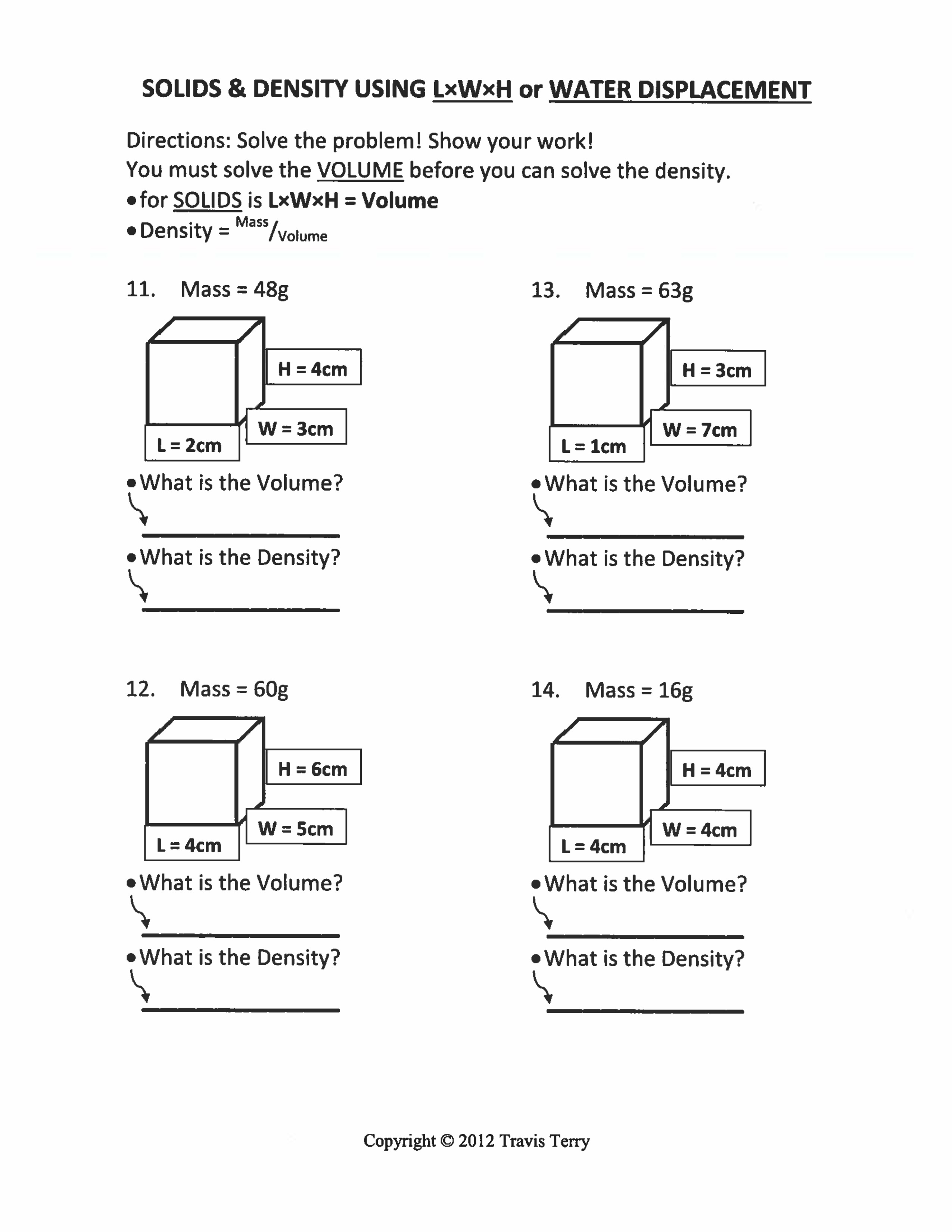
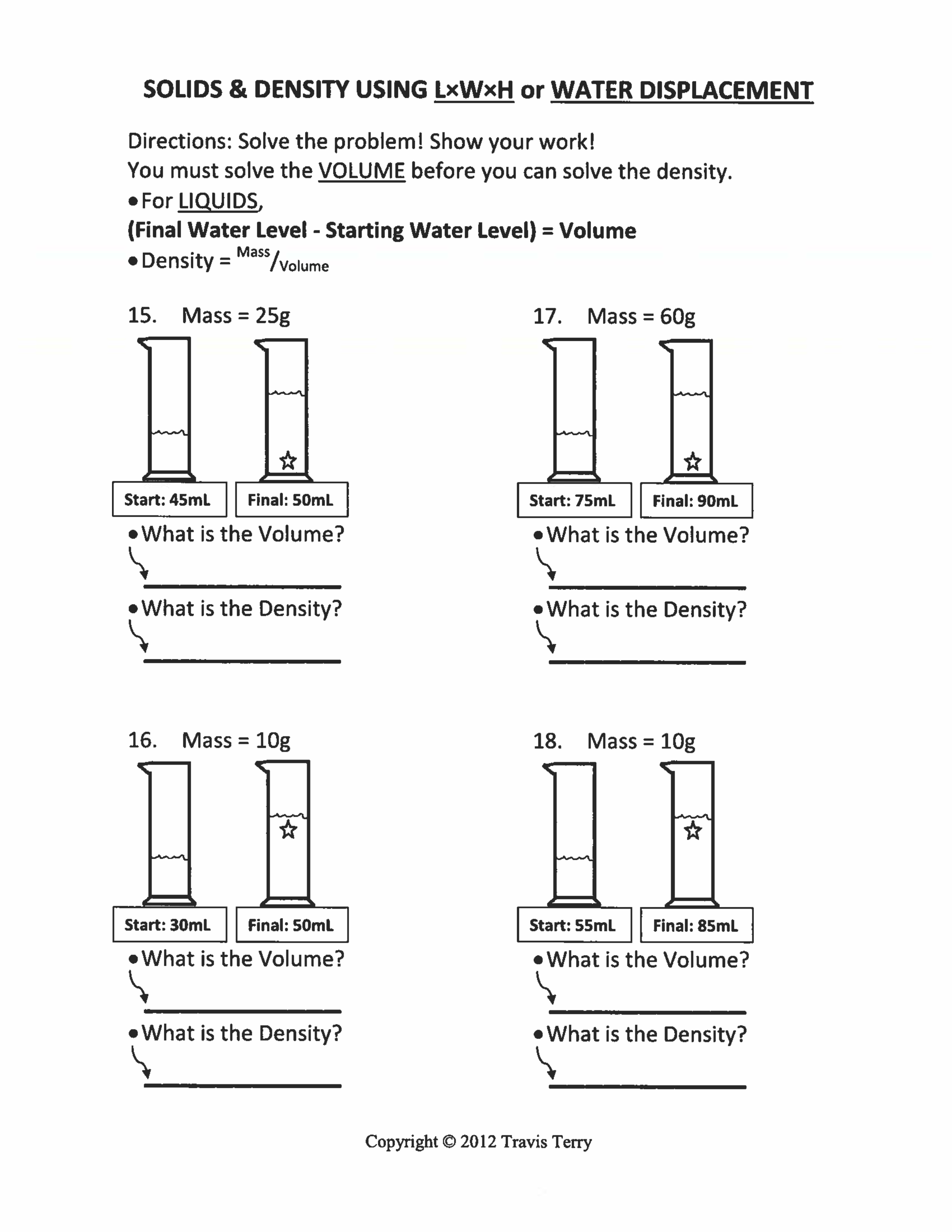
Density Test
star
star
star
star
star
Last updated over 1 year ago
30 questions
Required
5
Required
5
Required
5
Required
1
Required
5
Required
2
Required
3
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
| Draggable item | arrow_right_alt | Corresponding Item |
|---|---|---|
Density | arrow_right_alt | The amount of matter that will fit into a given amount of space |
Volume | arrow_right_alt | The amount of matter in something |
Mass | arrow_right_alt | The amount of space that an object occupies |
Miscible Liquids | arrow_right_alt | Liquids that do not mix together |
Immiscible Liquids | arrow_right_alt | Liquids that are able to mix together |
Meniscus | arrow_right_alt | The curved surface of the liquid in the graduated cylinder |