G11 Rates of reactions E-day Project
star
star
star
star
star
Last updated about 1 year ago
37 questions
Note from the author:
E-DAY PROJECT ON RATES OF REACTIONS
Open your textbook to the the start of Rates of Reactions. Follow along in your textbook as you work through this Formative.
You have two hours to complete this project. No reading time is necessary. After two hours, if you are still writing, Formative will force submit your answers (don't worry you won't lose the answers you have filled in if it submits automatically). If the power goes off and there is no wifi, I will ensure that your assessment is paused. Good luck!
E-DAY PROJECT ON RATES OF REACTIONS
Open your textbook to the the start of Rates of Reactions. Follow along in your textbook as you work through this Formative.
You have two hours to complete this project. No reading time is necessary. After two hours, if you are still writing, Formative will force submit your answers (don't worry you won't lose the answers you have filled in if it submits automatically). If the power goes off and there is no wifi, I will ensure that your assessment is paused. Good luck!
Using graphs to determine the rate of a reaction
Required
1
1
1
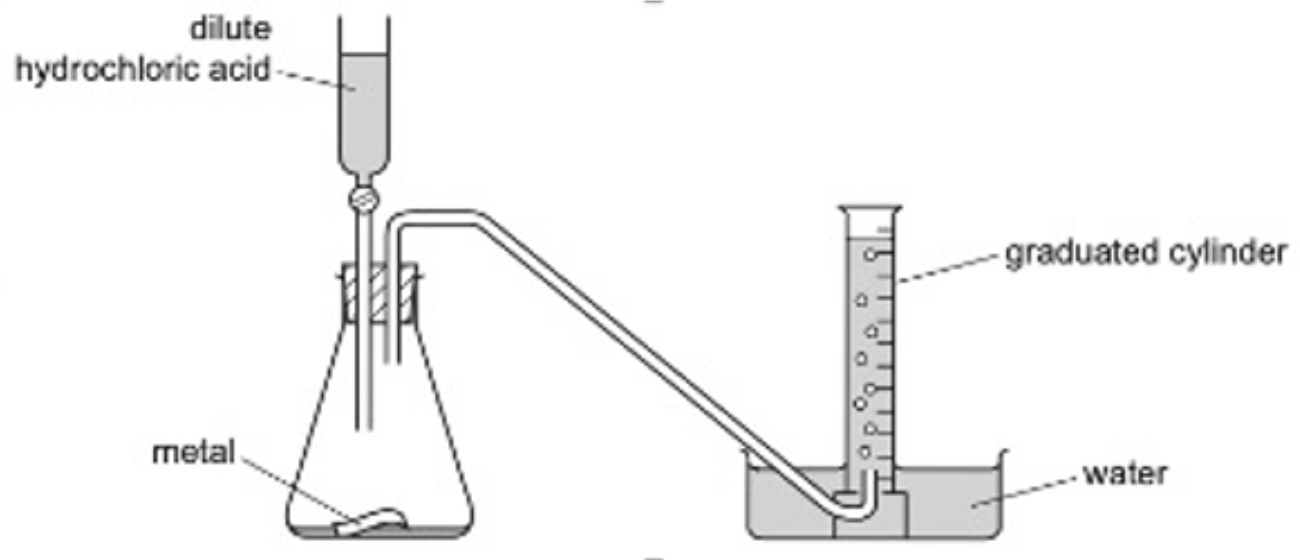
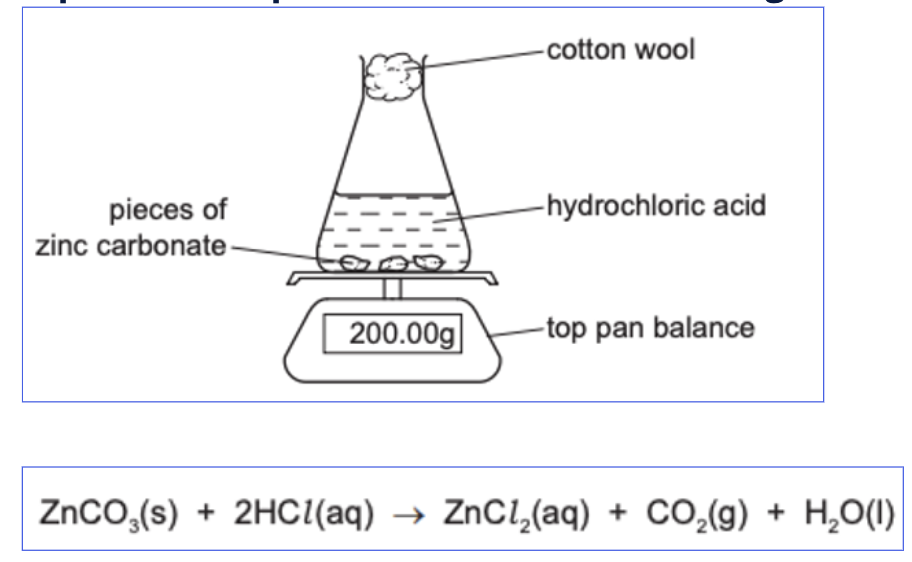
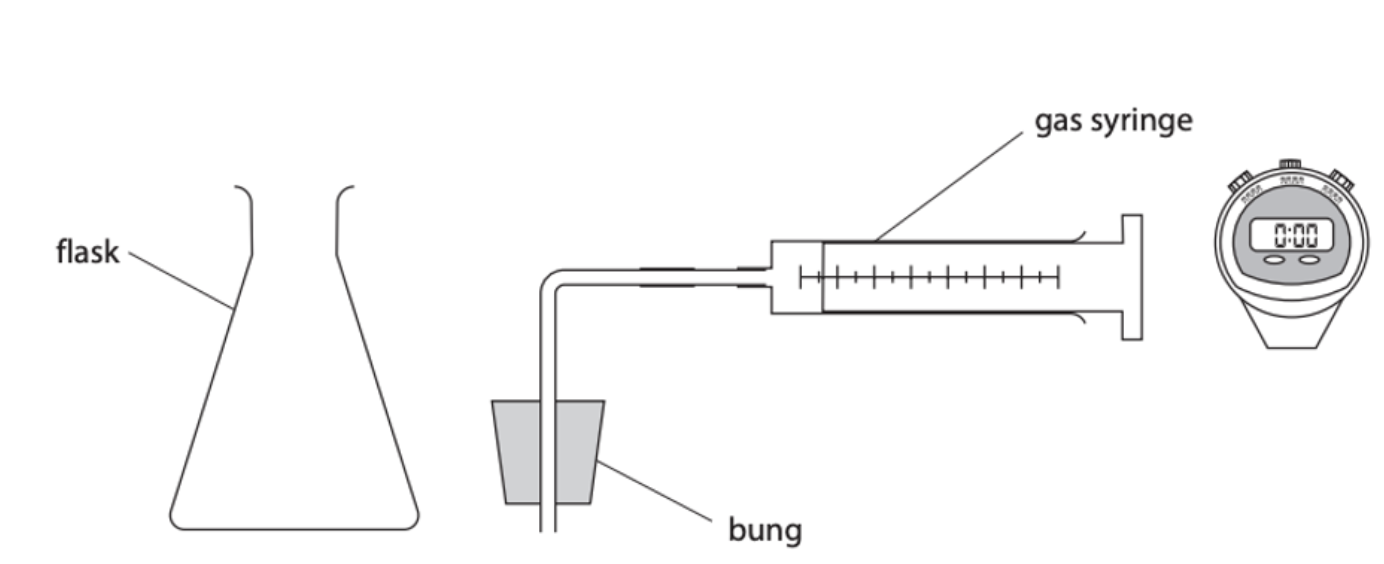
Measuring the rate of reaction in the laboratory
2
1
1
2
Required
1
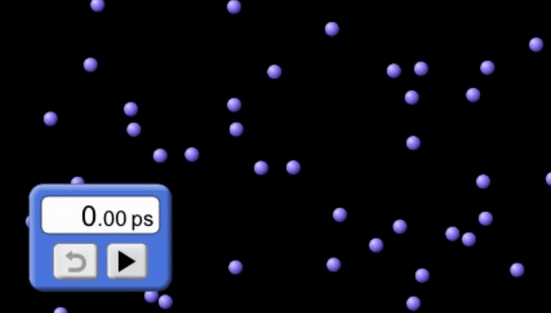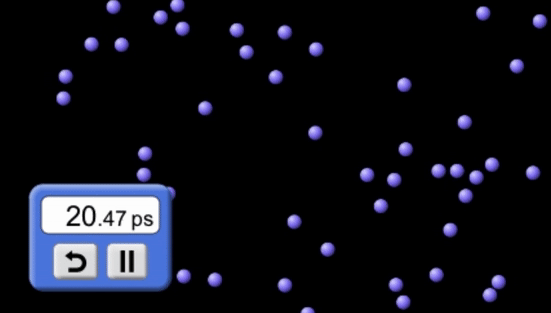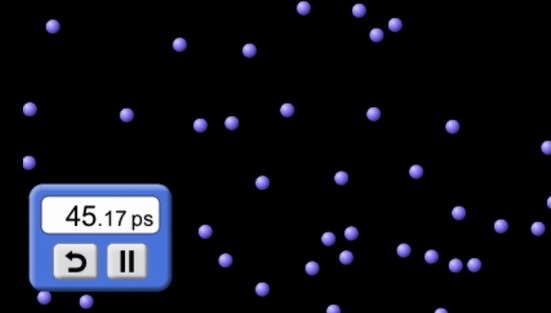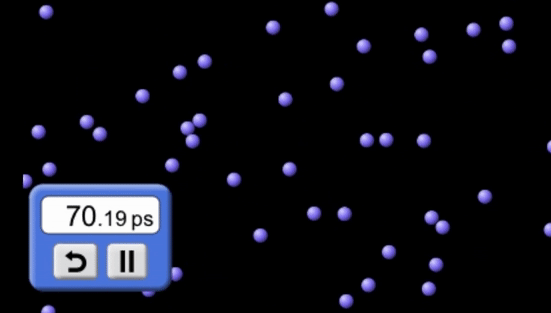
The factors that affect the rate of a reaction
1
1
1
1
1
1
Required
1
Required
1
General questions on rates of reactions
2
2
1
General laboratory skills
2
2
1