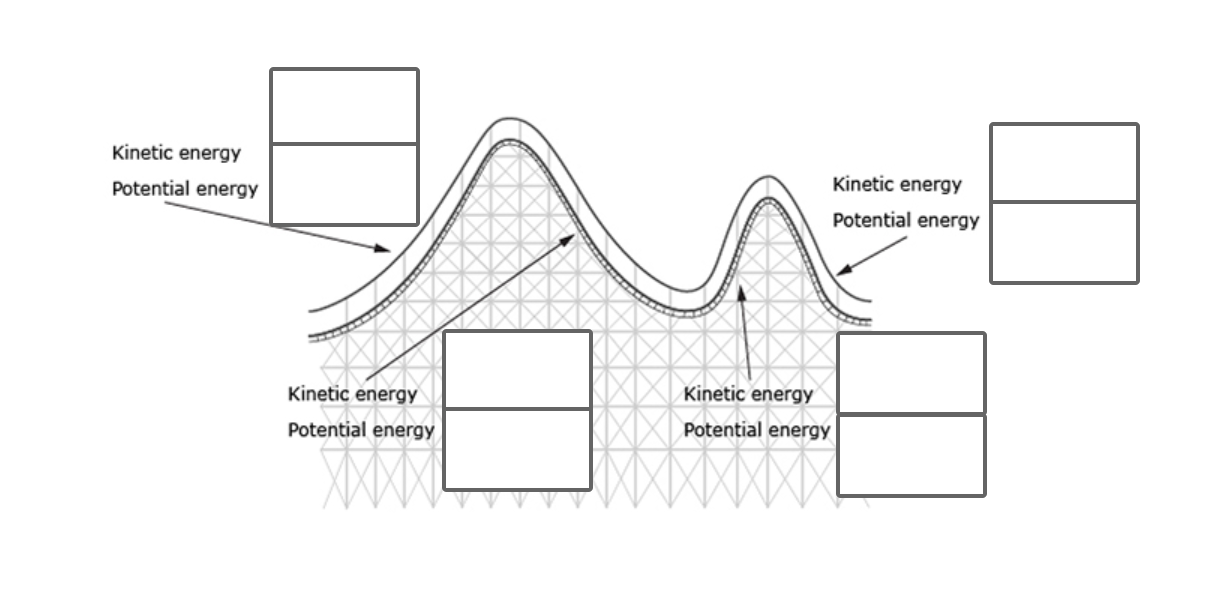
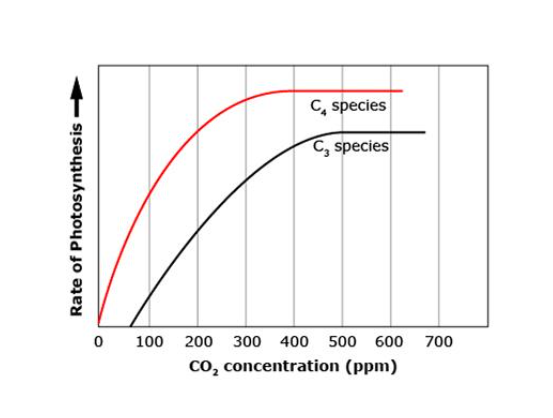
In comparing these two species, photosynthesis increases more rapidly in the presence of carbon dioxide for __________ plants. The C4 species reaches a peak rate of photosynthesis at __________and the C3 species reaches a peak rate of photosynthesis at __________. This means that carbon dioxide concentrations in excess of __________ will not increase the rate of plant growth or carbohydrate production for either species. The tropical C4 species grows more quickly. Since the photosynthesis reaction requires an equal number of CO2 and __________, this is adaptive for tropical environments.