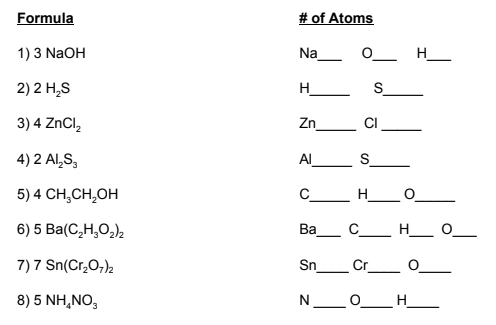
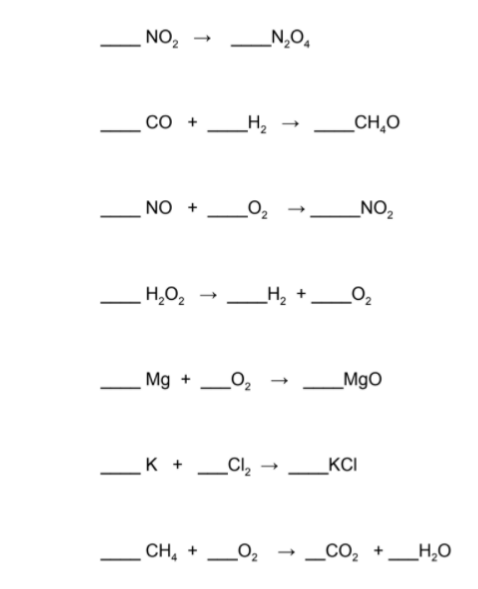
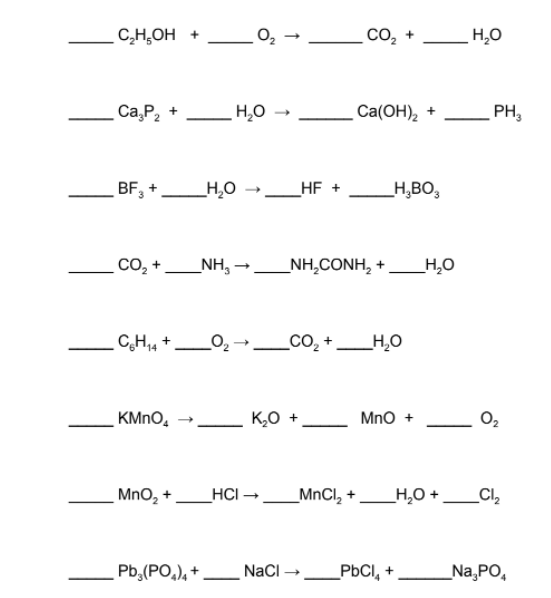
Now that you've been introduced to balancing equations, it's time to do some practice. The key thing to remember about balancing chemical equations is that it is a trial and error process. Don't be afraid to try something, erase it, try something else, erase that, try another thing - you get the picture.