When we study the trends in the periodic table, we cannot stop at just atomic size. In this section of the chapter, we will begin an understanding of an important concept, namely ionization energy and recognize its trend on the periodic table.
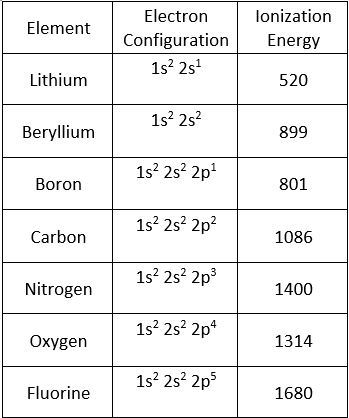
Lithium has an electron configuration of 1s22s1. Lithium has one electron in its outermost energy level. In order to remove this electron, energy must be added to the system. This energy is known as the ionization energy. The ionization energy is the energy required to remove the most loosely held electron from atom. The higher the value of the ionization energy, the harder it is to remove that electron.
We can see a trend when we look at the ionization energies for the elements in period 2. Look at the table below which shows the ionization energy for element in period 2.