Homework: The Carbon Cycle "Slow and Fast"
star
star
star
star
star
Last updated about 1 year ago
34 questions
Note from the author:
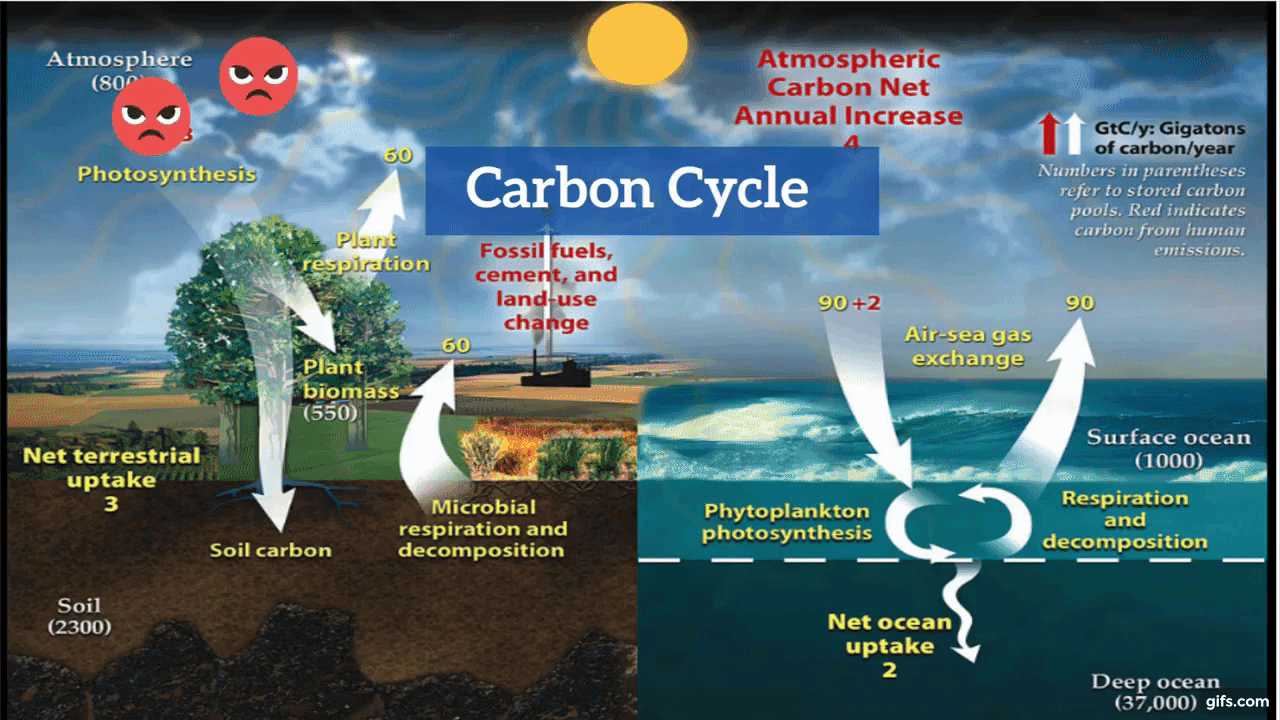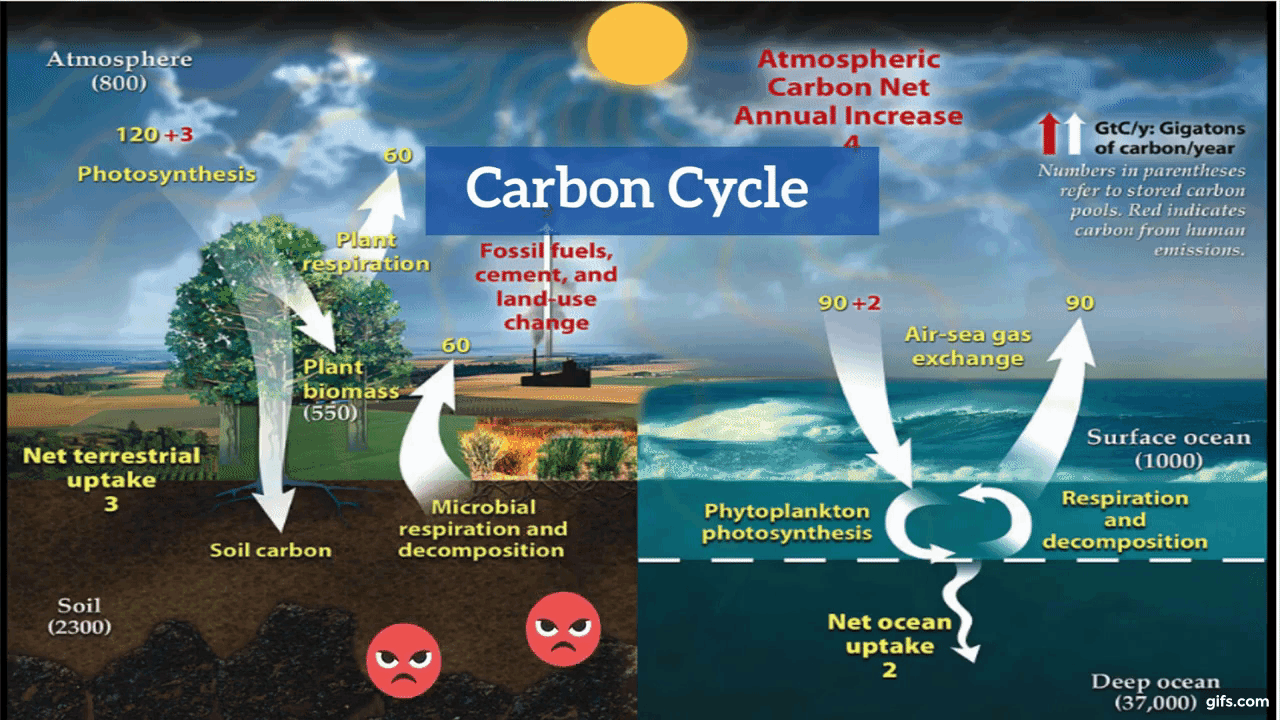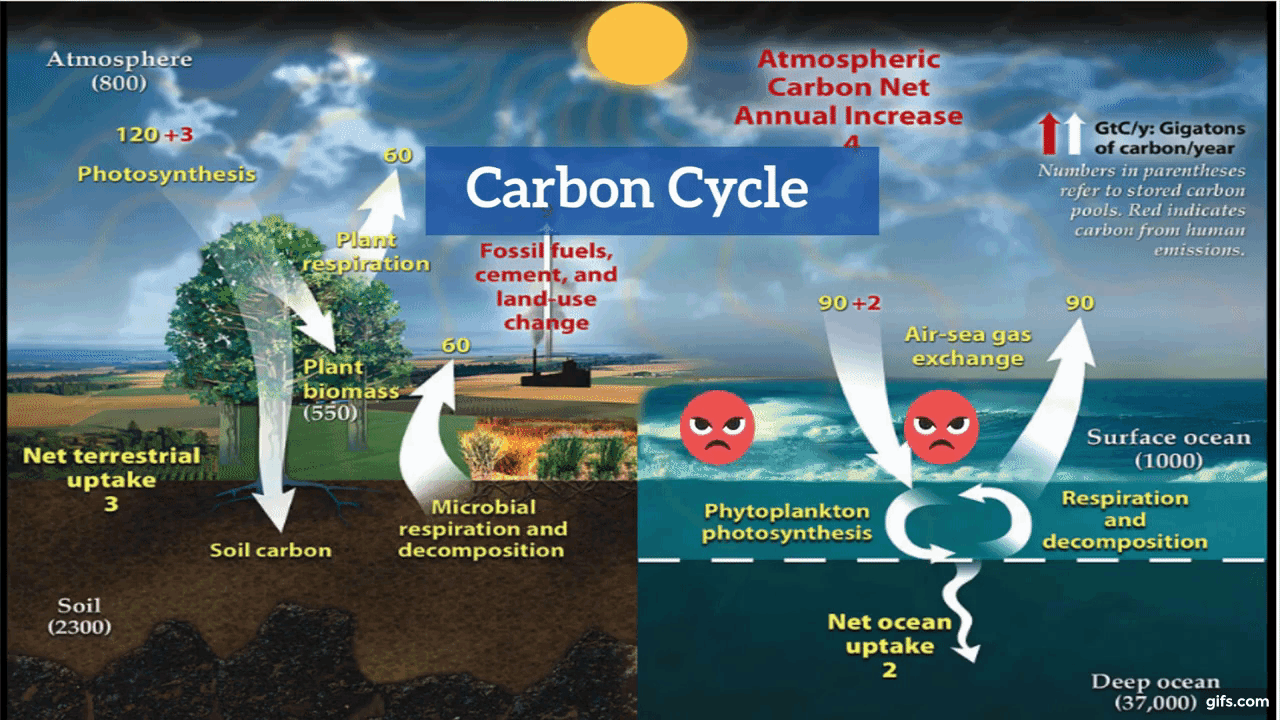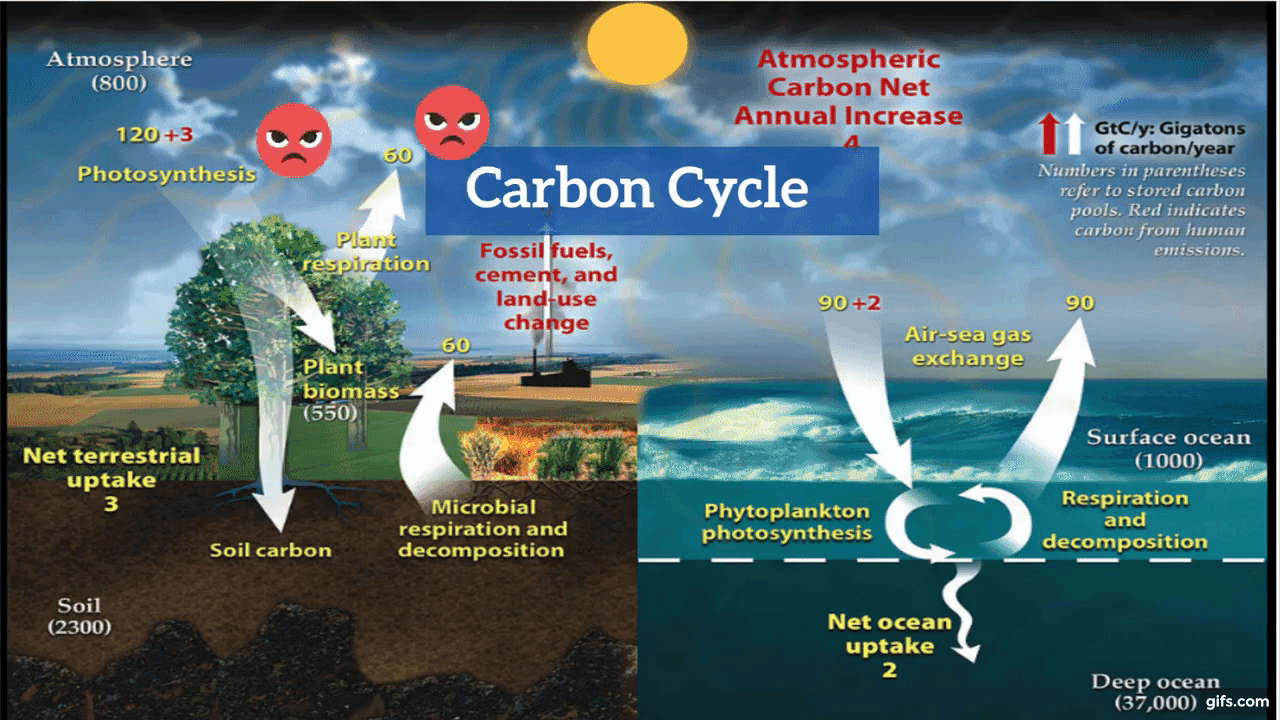
Carbon is the backbone of life on Earth. We are made of carbon, we eat carbon, and our civilizations—our economies, our homes, our means of transport—are built on carbon. We need carbon, but that need is also entwined with one of the most serious problems facing us today: global climate change.
Carbon is both the foundation of all life on Earth, and the source of the majority of energy consumed by human civilization. [Photographs ©2007 MorBCN (top) and ©2009 sarahluv (lower).]
Carbon is the backbone of life on Earth. We are made of carbon, we eat carbon, and our civilizations—our economies, our homes, our means of transport—are built on carbon. We need carbon, but that need is also entwined with one of the most serious problems facing us today: global climate change.
Carbon is both the foundation of all life on Earth, and the source of the majority of energy consumed by human civilization. [Photographs ©2007 MorBCN (top) and ©2009 sarahluv (lower).]
10
1