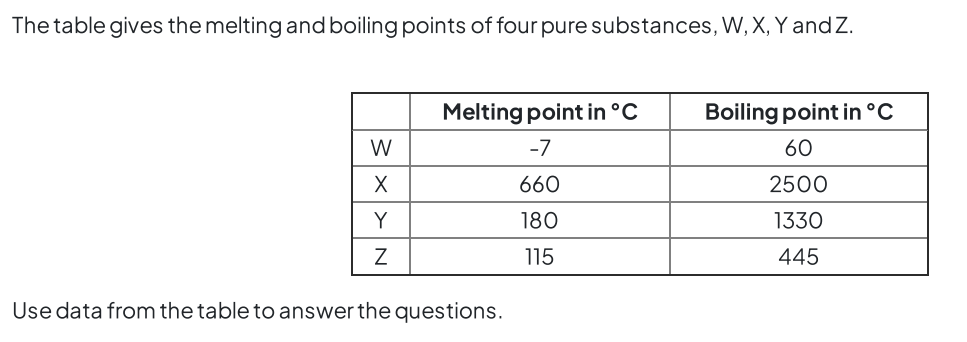
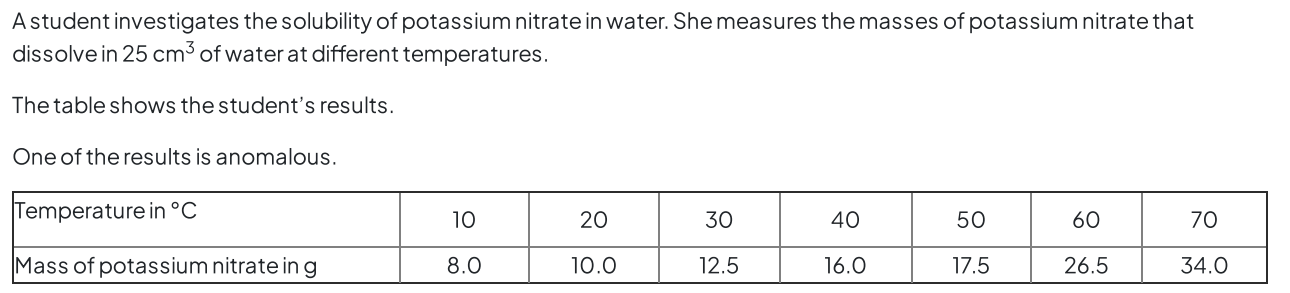
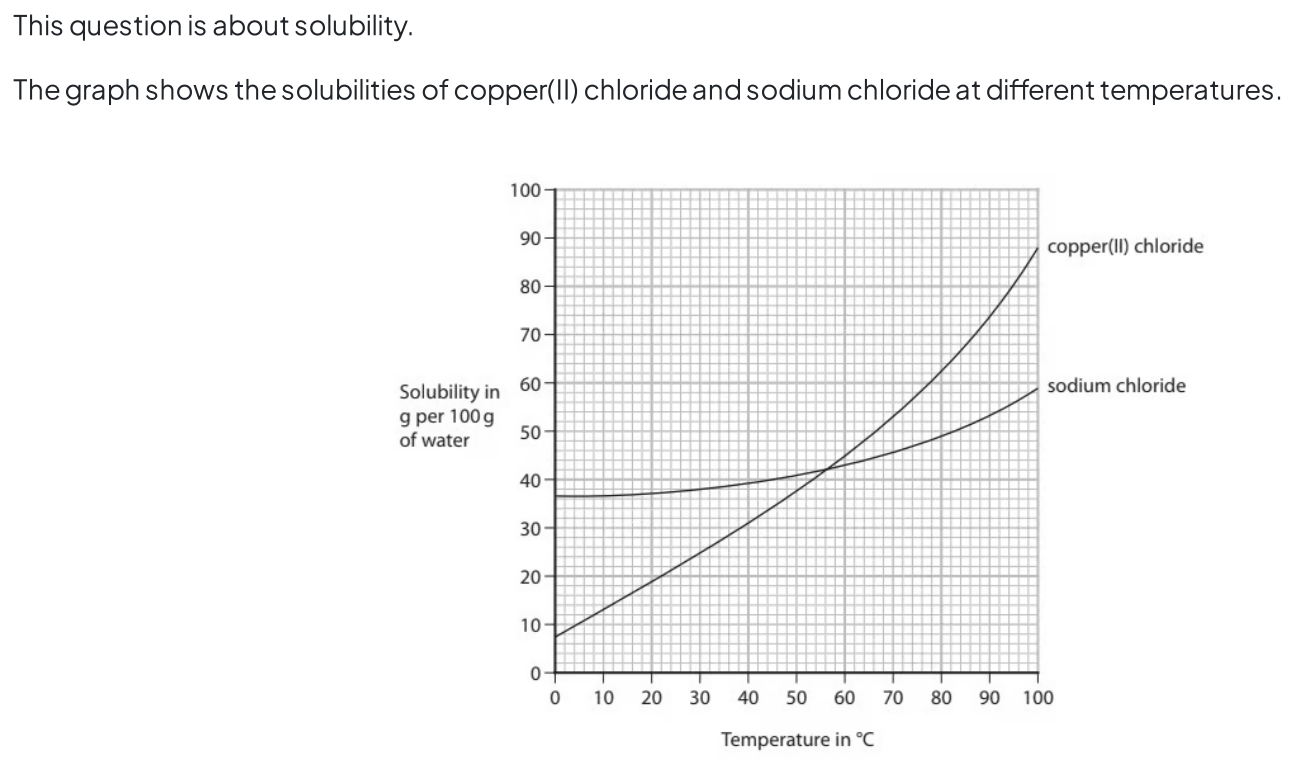
Topic 1 States of matter C (solubility)
star
star
star
star
star
Last updated 4 months ago
14 questions
2
3
Explain why solid lead iodide takes less time to form when the reaction is repeated using water at a higher temperature.
Explain why solid lead iodide takes less time to form when the reaction is repeated using water at a higher temperature.