3 Classifying Matter
star
star
star
star
star
Last updated 3 months ago
16 questions
Note from the author:
Learning Objectives
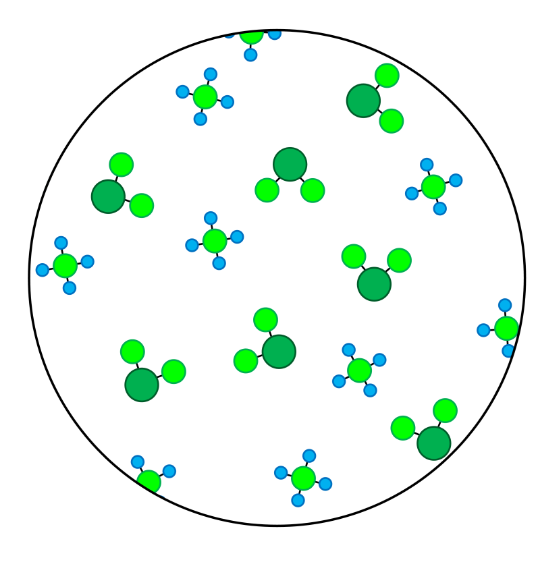
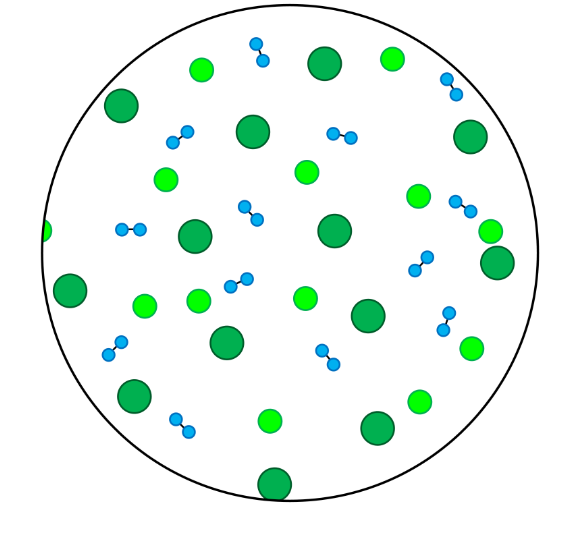
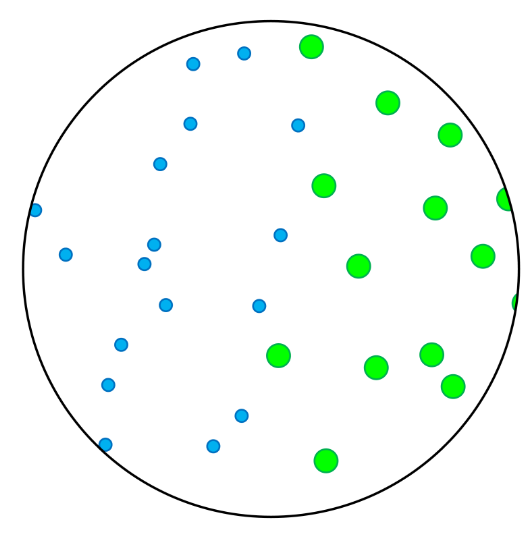
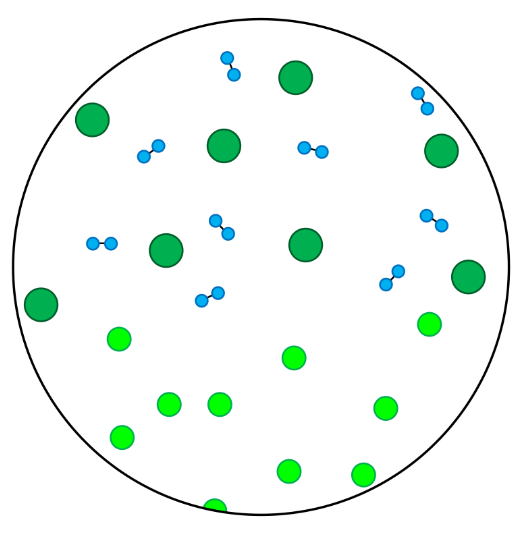
- In this skill we will learn to classify particles as elements or compounds, and we will learn to classify substances as either pure substances or mixtures.
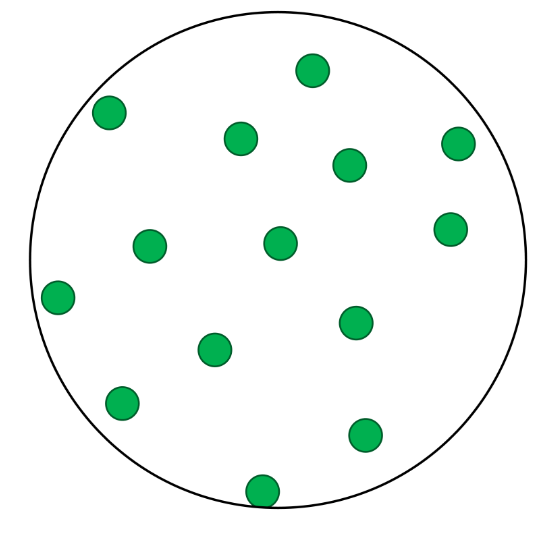
- Elements contain only one type of atom.
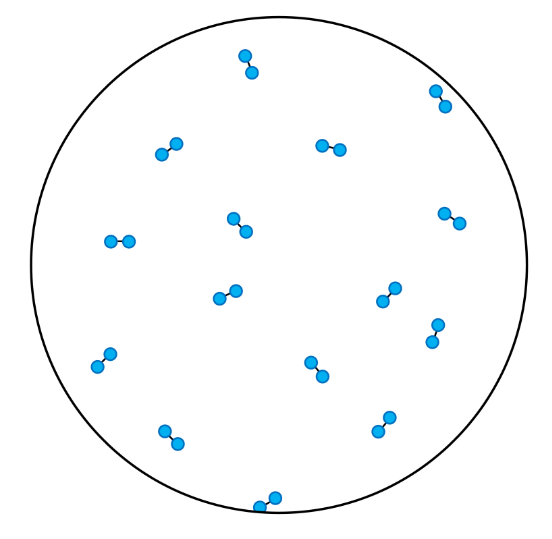
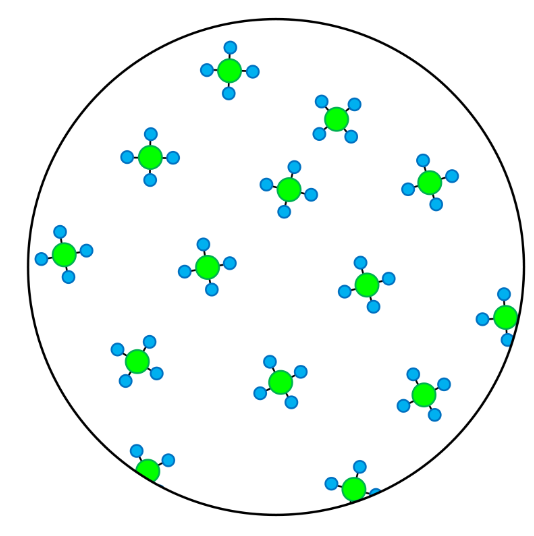
- Compounds contain atoms of more than one element that are chemically joined together.
- A pure substance is made up of one type of particle only - either an element or a compound.
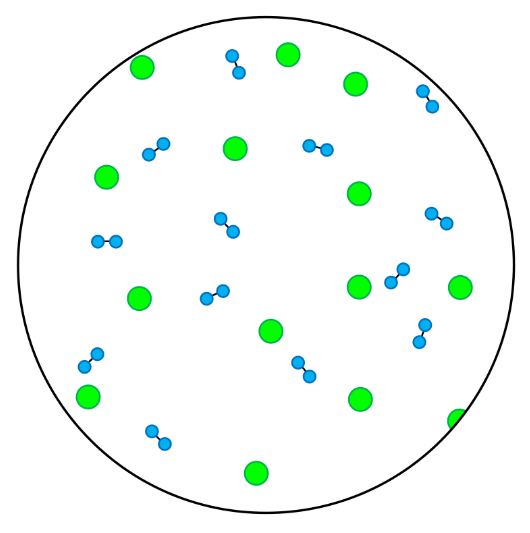
- A mixture is made up of more than one type of particle - this could be a mixture of elements, a mixture of compounds, or a mixture of elements and compounds.
- Homogenous mixtures are mixtures in which the different types of particles are evenly spread throughout.
- Heterogenous mixtures are mixtures in which the different types of particles are not evenly spread throughout.
Tips
- In these questions, different elements are shown by circles of different sizes and colors.
- Elements can be monatomic (made up of just one atom) or diatomic (made up of two atoms chemically joined together).
Learning Objectives
- In this skill we will learn to classify particles as elements or compounds, and we will learn to classify substances as either pure substances or mixtures.
- Elements contain only one type of atom.
- Compounds contain atoms of more than one element that are chemically joined together.
- A pure substance is made up of one type of particle only - either an element or a compound.
- A mixture is made up of more than one type of particle - this could be a mixture of elements, a mixture of compounds, or a mixture of elements and compounds.
- Homogenous mixtures are mixtures in which the different types of particles are evenly spread throughout.
- Heterogenous mixtures are mixtures in which the different types of particles are not evenly spread throughout.
Tips
- In these questions, different elements are shown by circles of different sizes and colors.
- Elements can be monatomic (made up of just one atom) or diatomic (made up of two atoms chemically joined together).