Quarter 1 Test
star
star
star
star
star
Last updated over 1 year ago
25 questions
Note from the author:
You may complete just the sections of the test that you wish to try and improve your grade on. You do not need to answer all the questions if you are not trying to raise your grades for certain learning targets.
You may complete just the sections of the test that you wish to try and improve your grade on. You do not need to answer all the questions if you are not trying to raise your grades for certain learning targets.
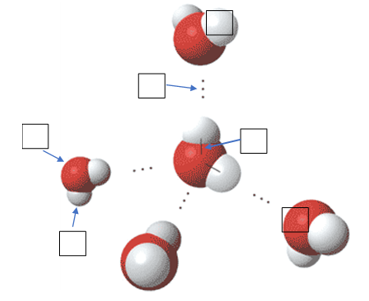
1.1a I can explain how water’s physical properties contribute to polarity, adhesion, cohesion, thermal regulation, density and solubility
1
1
1
1
1
1.1b I can relate the chemical and physical properties of water to life’s metabolic activities
1
1
1
1
1
1.2a – I can recognize that living cells are composed of relatively few elements including carbon (C), hydrogen (H), nitrogen (N), oxygen (O), phosphorous (P), and sulfur (S).d Section
1
1
1
1
1
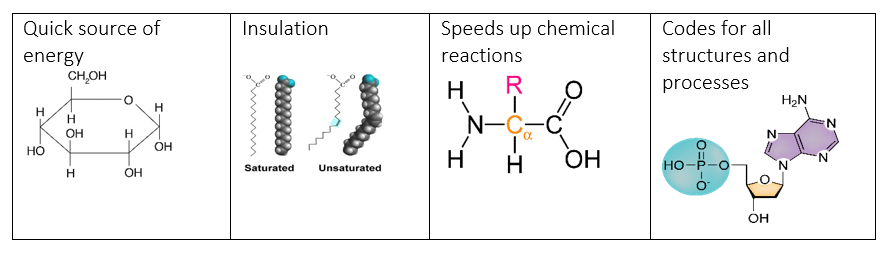
1.2b - I can differentiate between the four major categories of macromolecules (lipids, carbohydrates, proteins, and nucleic acids) through their primary roles and functions.
1
1
1
1
1
1.3a I can describe the structure of enzymes and explain their in role in acting as catalysts to control the rate of metabolic reactions
1
1
1
1
1