Thermodynamics Principles
star
star
star
star
star
Last updated over 2 years ago
10 questions
Unit B: Energy Flow and Technological Systems.
STS Outcome 3: Apply the principles of energy conservation and thermodynamics to investigate, describe, and predict the efficiency of energy transformation in technological systems
- 3.1 Describe, qualitatively and in terms of thermodynamic laws, the energy transformations occurring in devices and systems
- 3.2 Describe how the first and second laws of thermodynamics have changed our understanding of energy conversions
- 3.4 Recognizes that there are limits to the amount of “useful” energy that can be derived from the conversion of potential energy to other forms in a technological device
- 3.6 Apply concepts related to the efficiency of thermal energy conversion to analyze the design of a thermal device
ICT Outcomes
- C6 Students will use technology to investigate and/or solve problems
4.4 generate new understandings of problematic situations by using some form of technology to facilitate the process
- C7 Students will use electronic research techniques to construct personal knowledge and meaning
4.2 analyze and synthesize information to determine patterns and links among ideas
- F4 Students will become discerning consumers of mass media and electronic information
4.2 evaluate the influence and results of digital manipulation on our perceptions
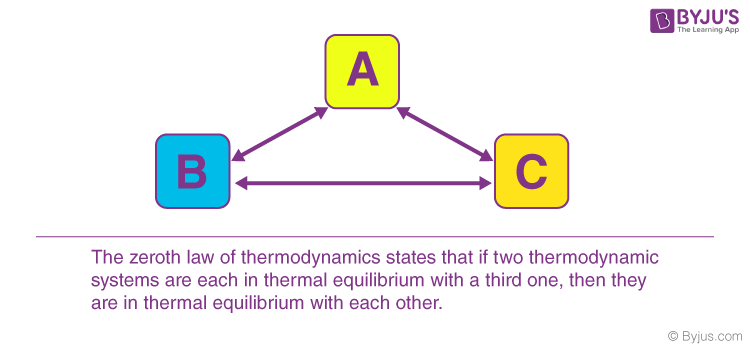
The Zeroth Law of Thermodynamics
The First Law of Thermodynamics
Required
5
The Second Law of Thermodynamics
Required
2
Required
2
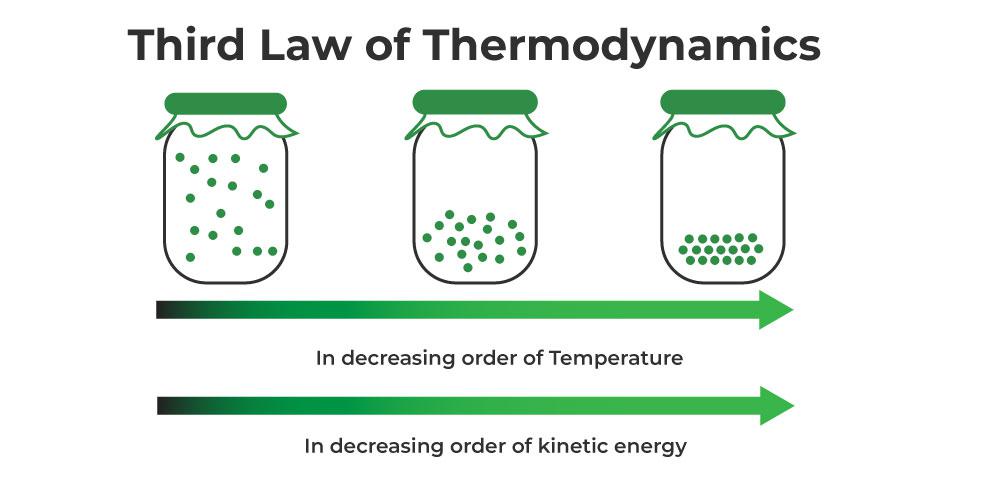
The Third Law of Thermodynamics
Required
4
Required
6