Week 8 Y12 EXP 2 Electronegativity
star
star
star
star
star
Last updated over 2 years ago
10 questions
Note from the author:
Week 8 Year 12 EXP 2
2
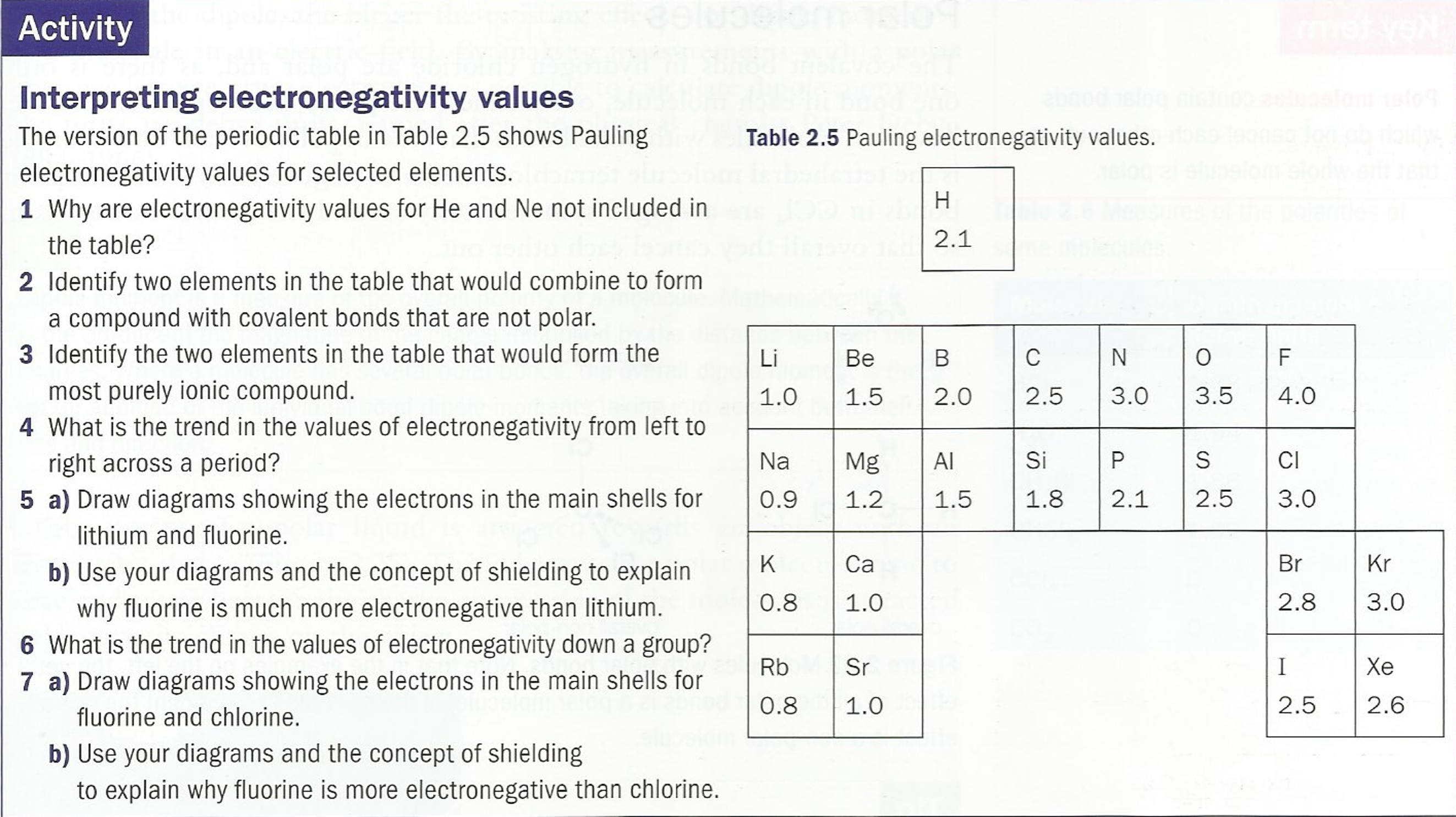
Suggest why the electronegativity of fluorine is greater than that of chlorine, despite the fact that the nucleus of a chlorine atom contains more protons
Suggest why the electronegativity of fluorine is greater than that of chlorine, despite the fact that the nucleus of a chlorine atom contains more protons
3
•2) Ionic bonding and covalent bonding are two extremes of chemical bonding; many compounds have bonding that is intermediate in character•(a) Giving an example in each case, explain what is meant by the terms ‘ionic bonding’ and ‘covalent bonding’•(b) Select a compound that has bonding of an intermediate character and explain why it has this type of bonding
•2) Ionic bonding and covalent bonding are two extremes of chemical bonding; many compounds have bonding that is intermediate in character
•(a) Giving an example in each case, explain what is meant by the terms ‘ionic bonding’ and ‘covalent bonding’
•(b) Select a compound that has bonding of an intermediate character and explain why it has this type of bonding
1