Osmosis Worksheet
star
star
star
star
star
Last updated about 2 years ago
31 questions
Osmosis Worksheet
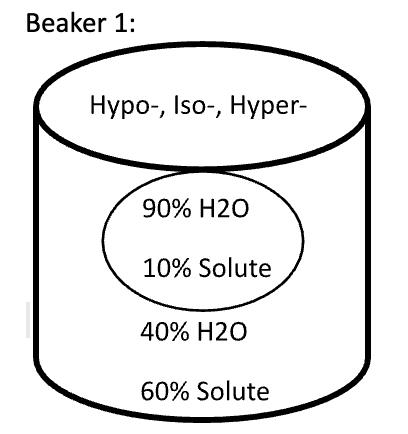
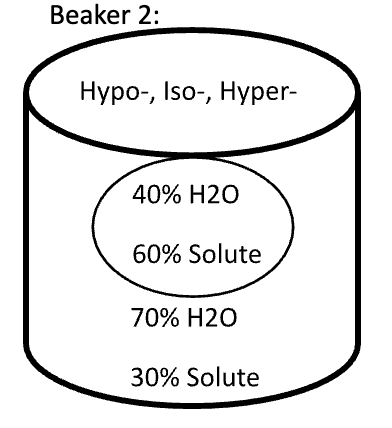
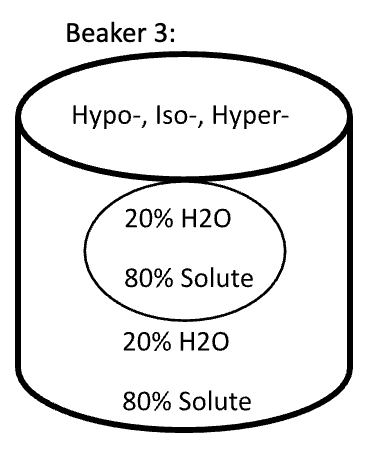
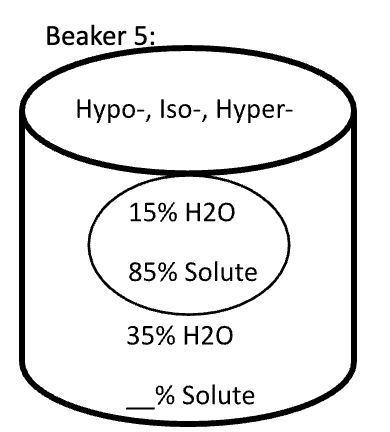
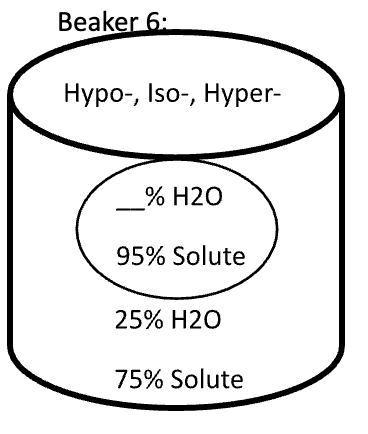
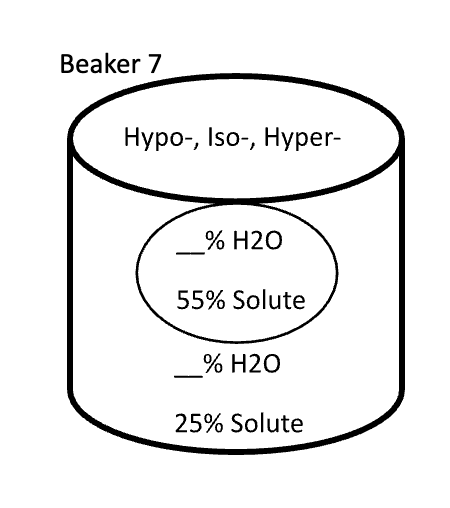
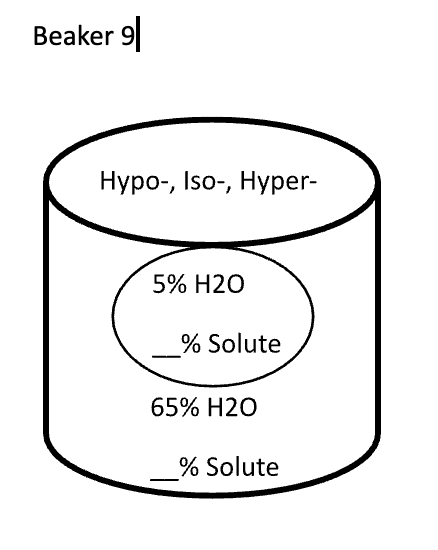
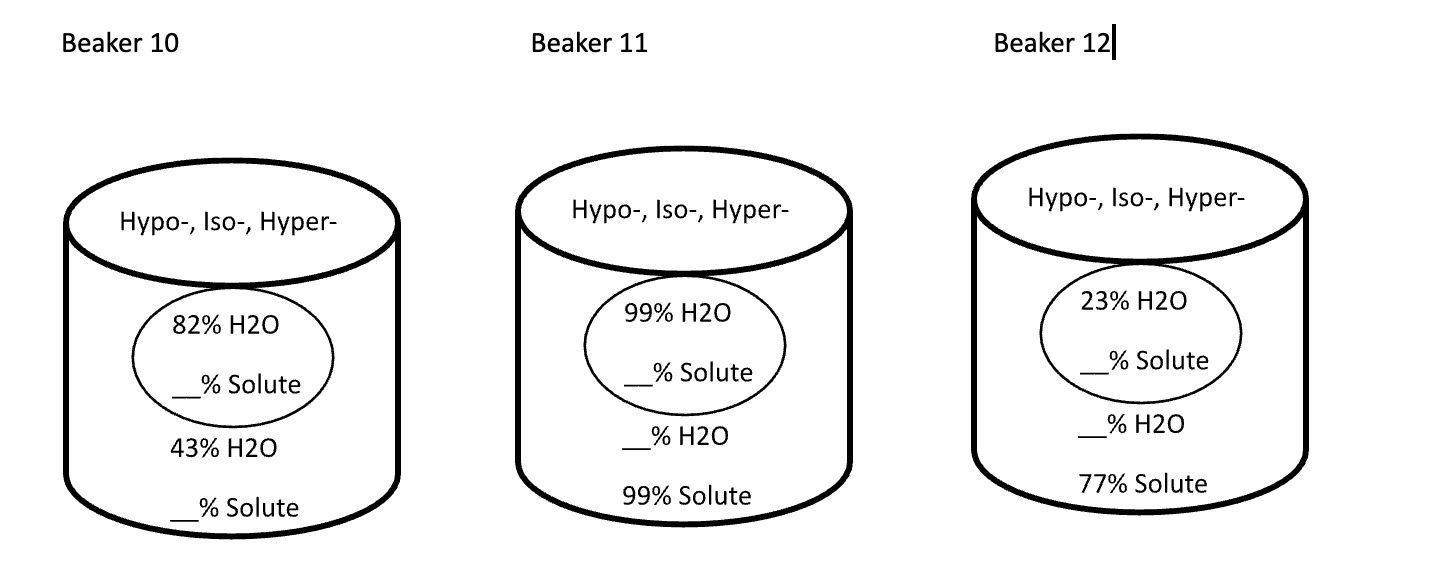
Below are animal cells placed in beakers of various concentrations. Use the first picture as an example.
- Select the direction that water moves (into the cell or into the solution)
- Fill in any missing percentages (water or solute)
- Identify the type of solution (isotonic, hypertonic, or hypotonic)
Required
1
Required
1