A student investigates the reaction between sodium hydroxide solution and dilute sulfuric acid.
He does a titration to find the concentration of the sulfuric acid.
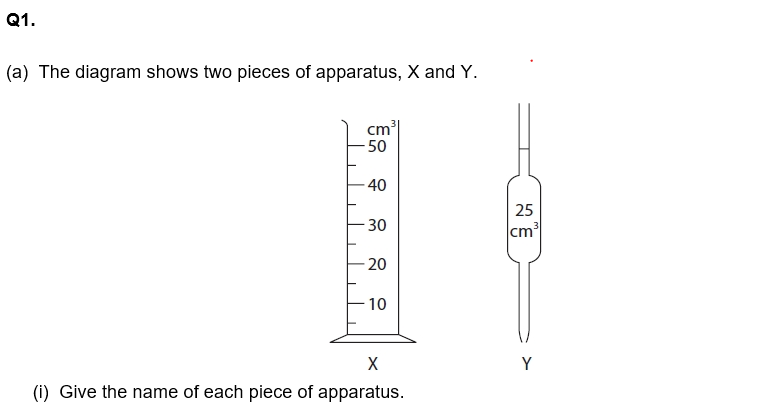
This is his plan for the titration. There are some mistakes and omissions in his plan.
· rinse a conical flask with the sodium hydroxide solution
· use a measuring cylinder to measure out 25 cm3 of the sodium hydroxide solution and add it to the conical flask
· add a few drops of methyl orange indicator to the conical flask
· rinse a burette with water and then fill it with the sulfuric acid
· add the acid from the burette to the conical flask until the indicator changes colour at the end-point of the titration
· record the final burette reading
(a) Give the colour change of the methyl orange indicator at the end-point.