CP Chemistry Semester 1 Midterm
star
star
star
star
star
Last updated over 1 year ago
35 questions
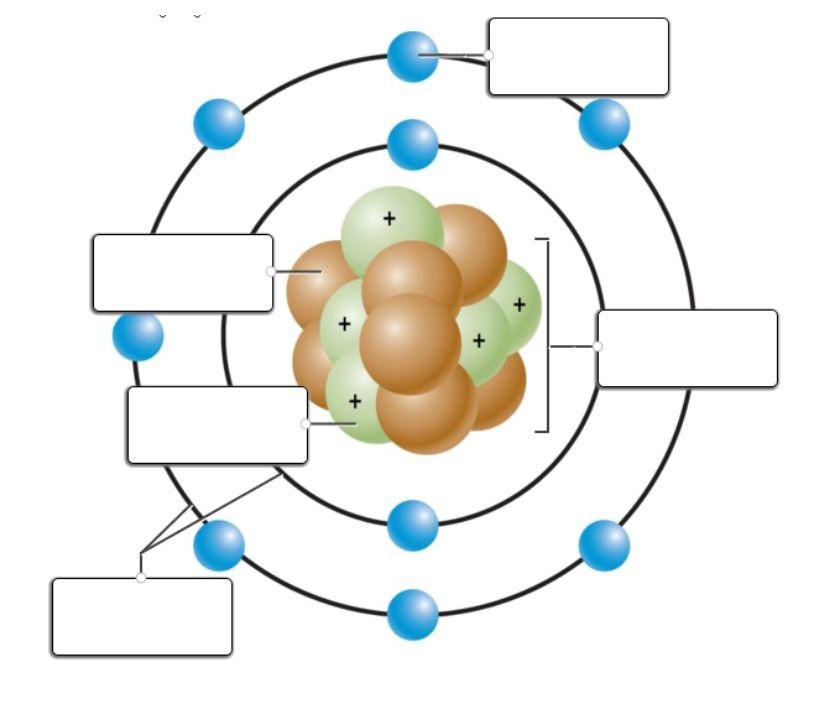
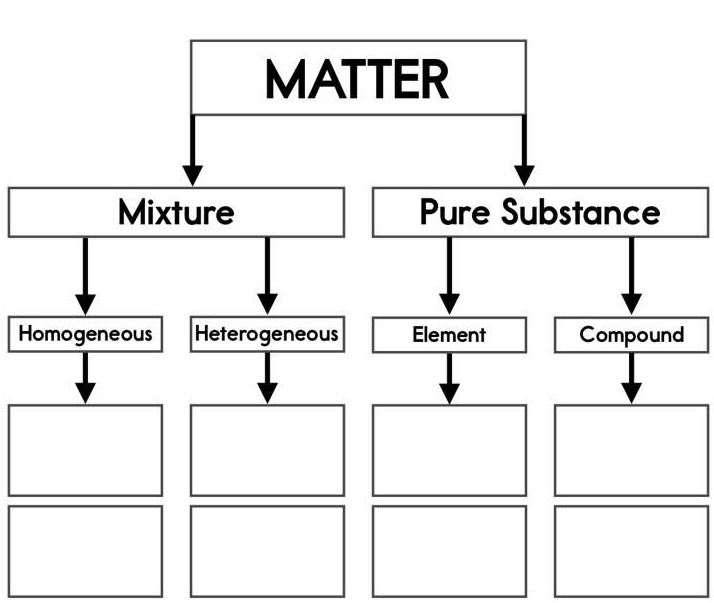
Read each question carefully and choose the best response. Use the periodic table below to help you as needed. If you click on it, it will enlarge on your screen.
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
1
Required
5
Required
8