Lewis Dot Diagrams (GRADED)
star
star
star
star
star
Last updated about 2 years ago
32 questions
Valence Electrons and Lewis Dot Diagram of period 2 elements.
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
Required
1
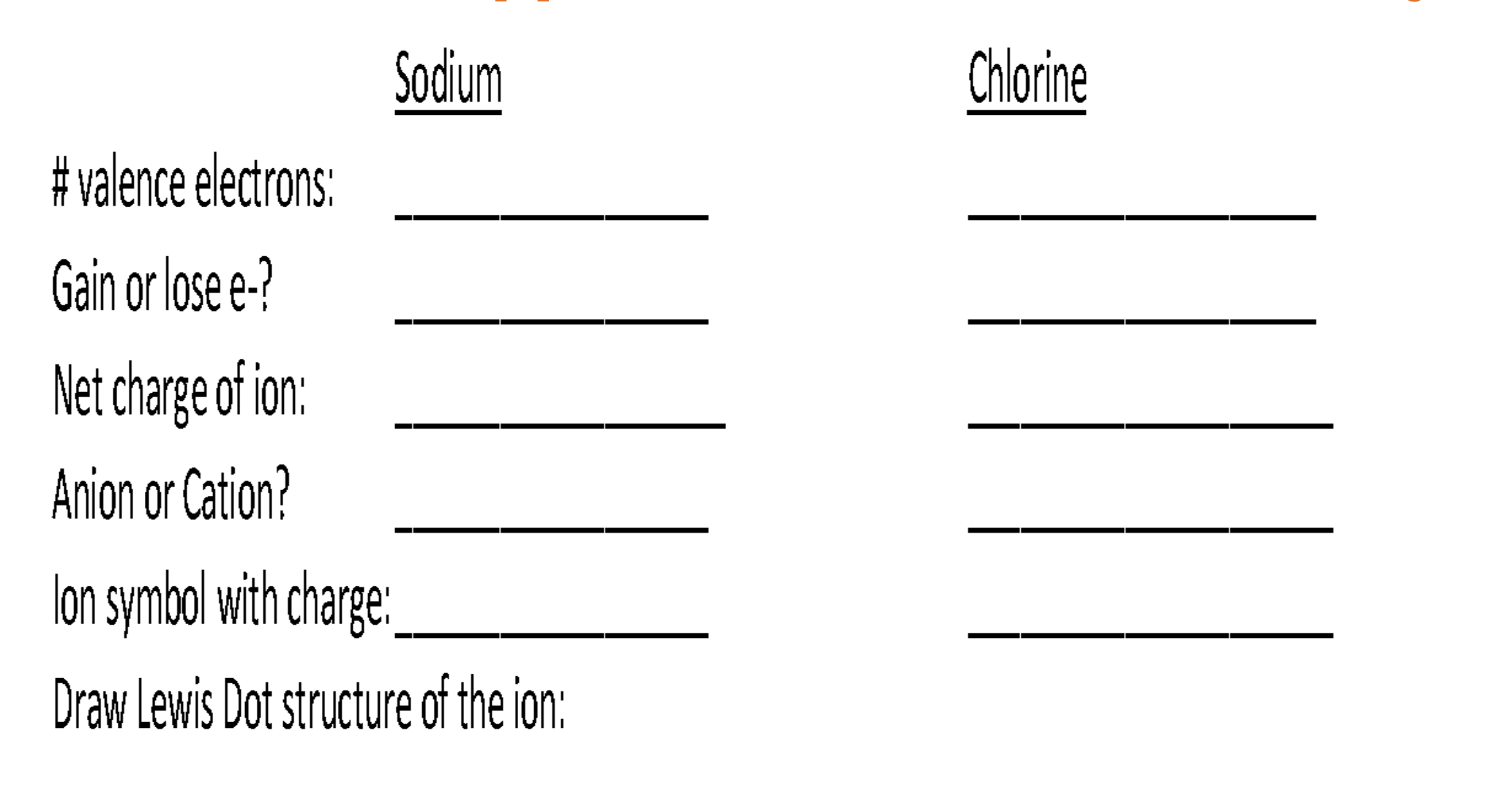
Octet Rule & Lewis dot diagram of ions
1
Required
1
Required
1
Ionic Compounds
1
Recap: Complete the questions 13 and 23.
1
1
1
1
Challenge Yourself
Required
1
1
1
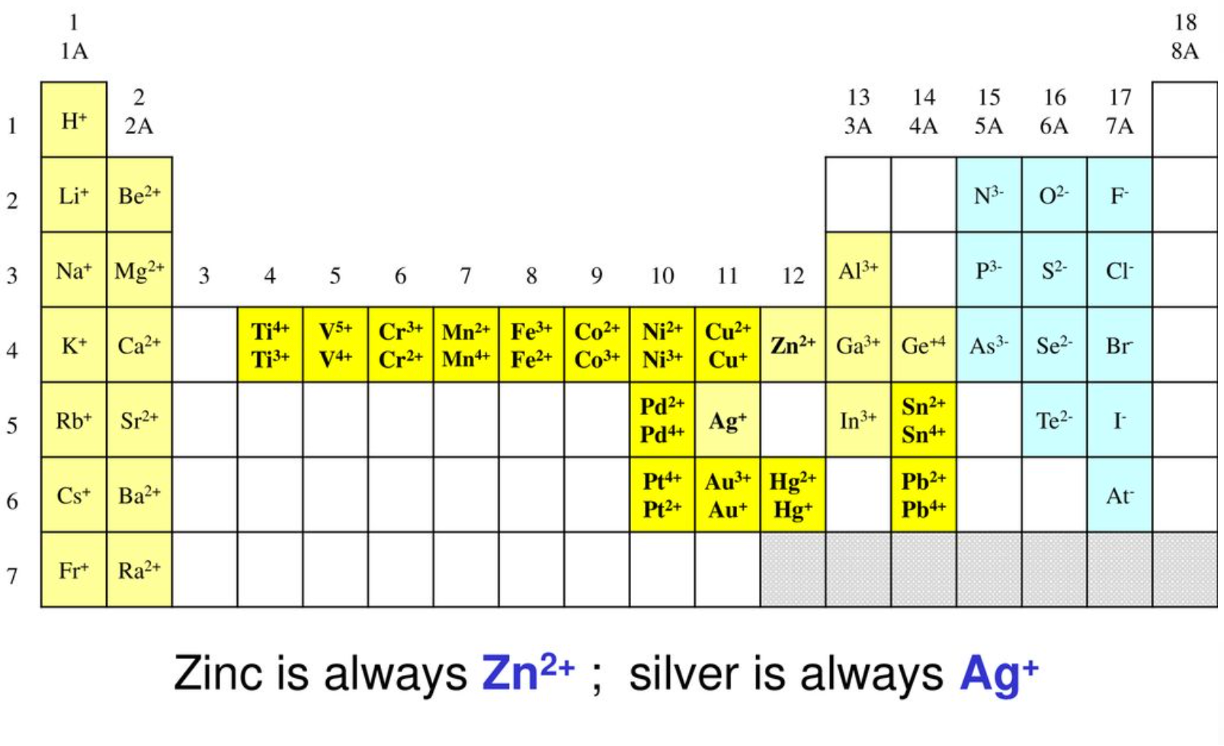
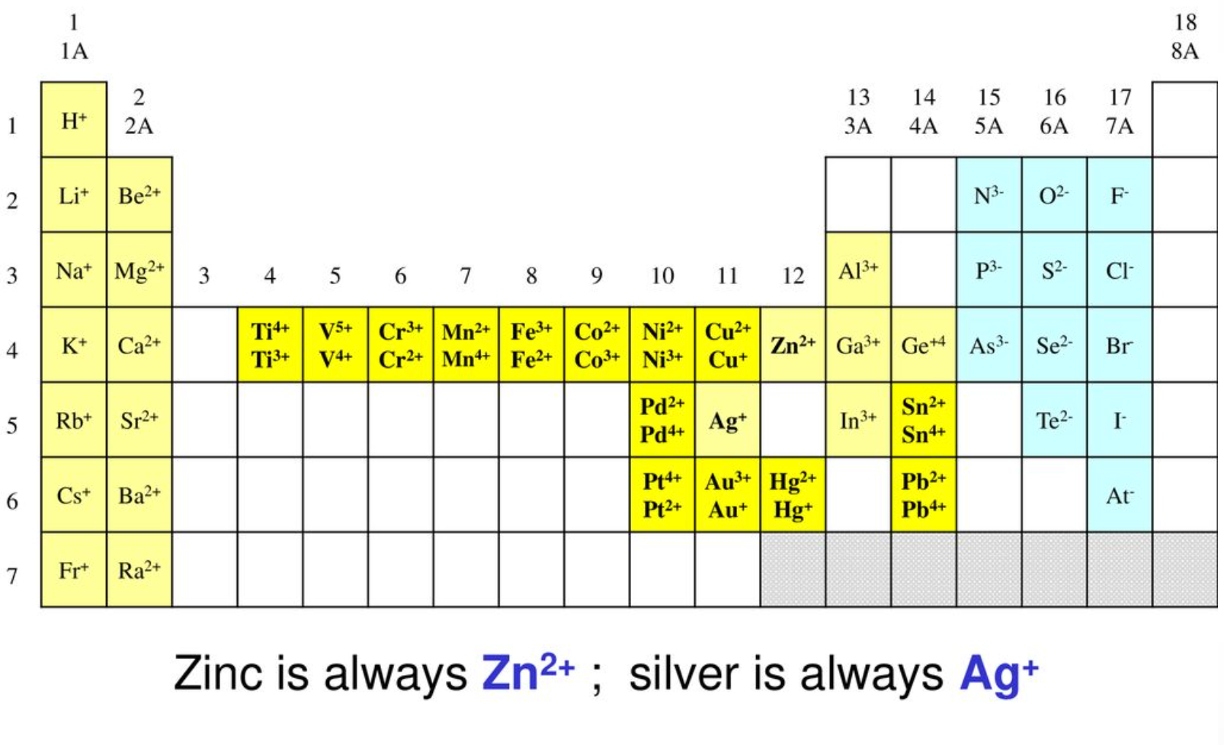
Transition metal cations. Use the image below to guide you complete this activity.
Required
1
Required
1