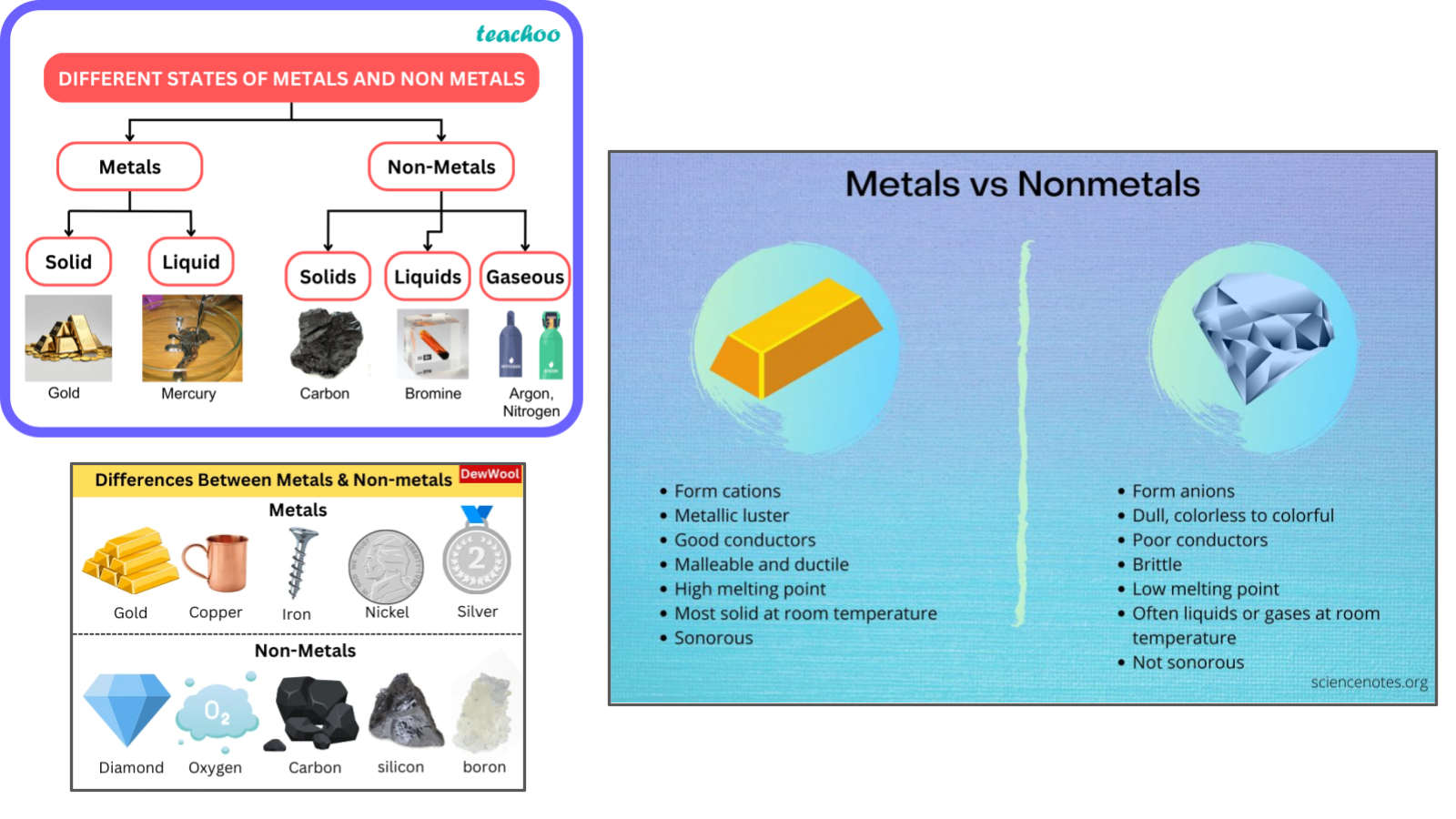
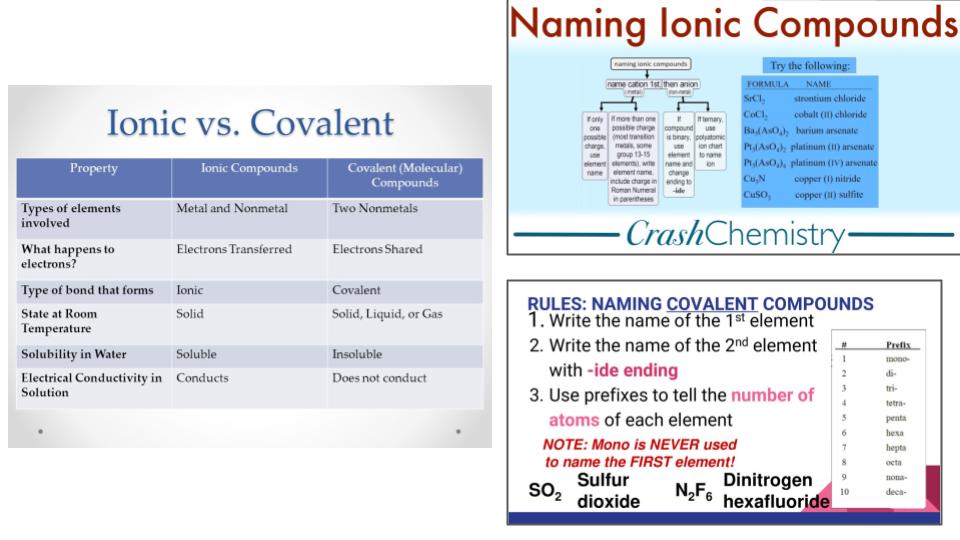
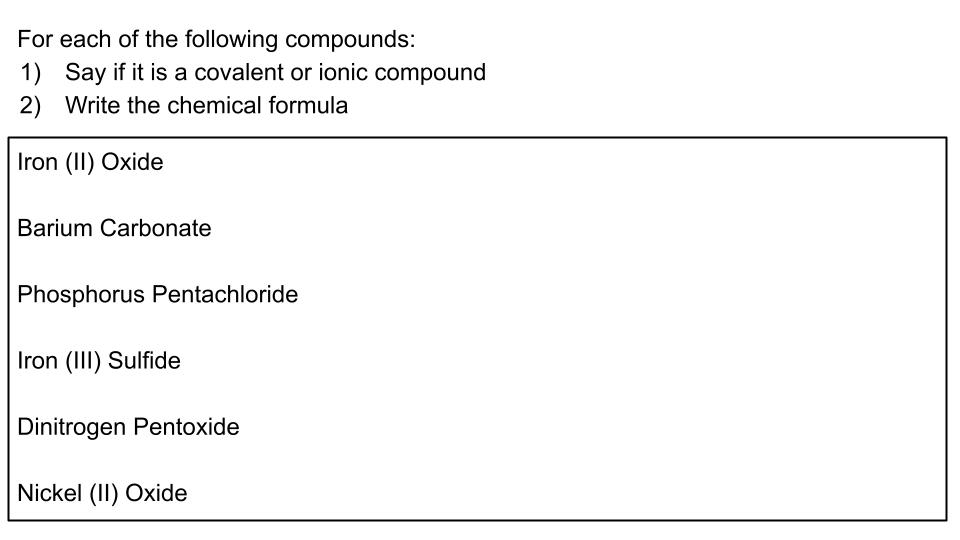
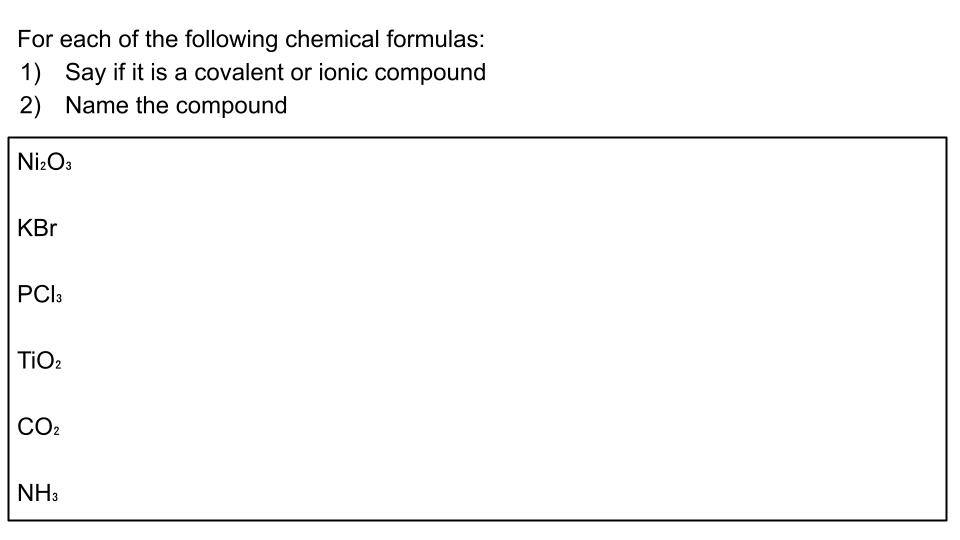
Unit 3 & 4 Review
star
star
star
star
star
Last updated about 2 years ago
30 questions
Note from the author:
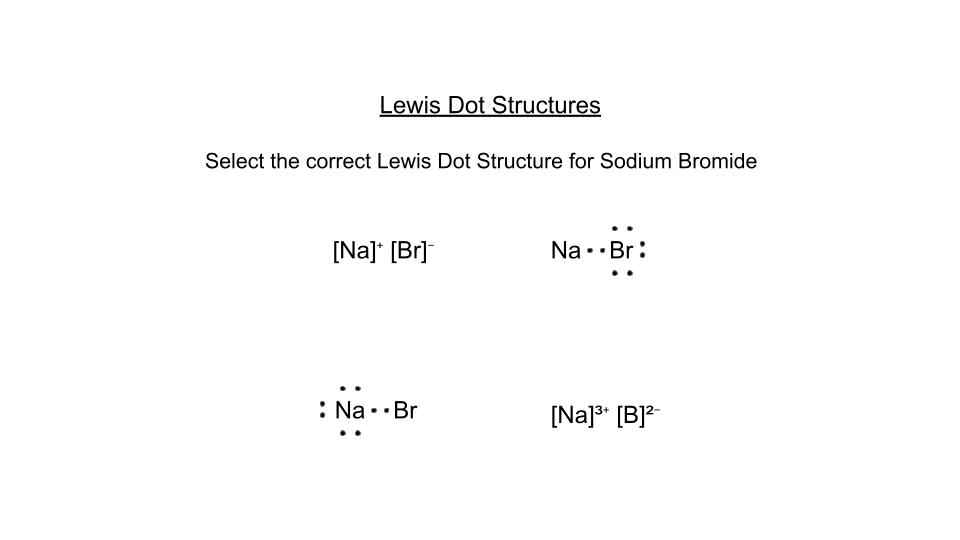
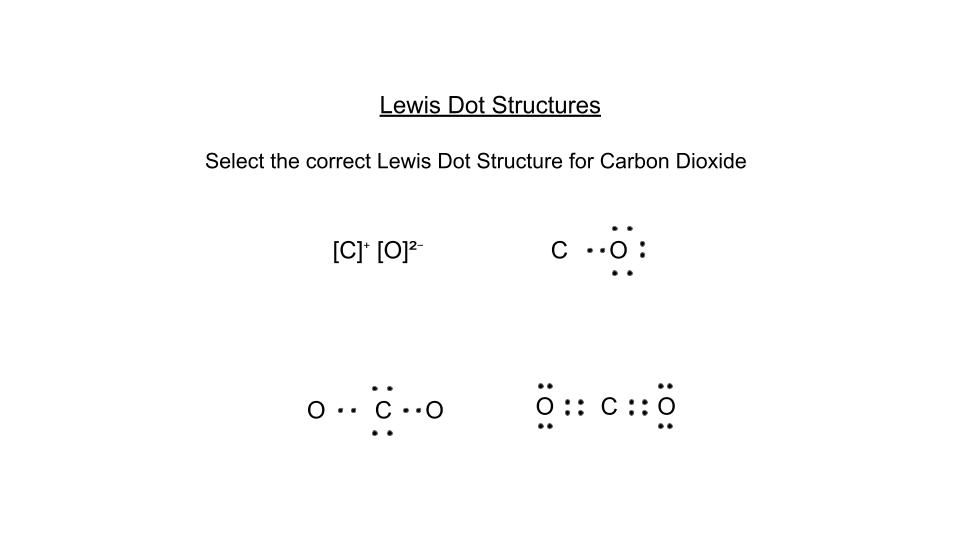
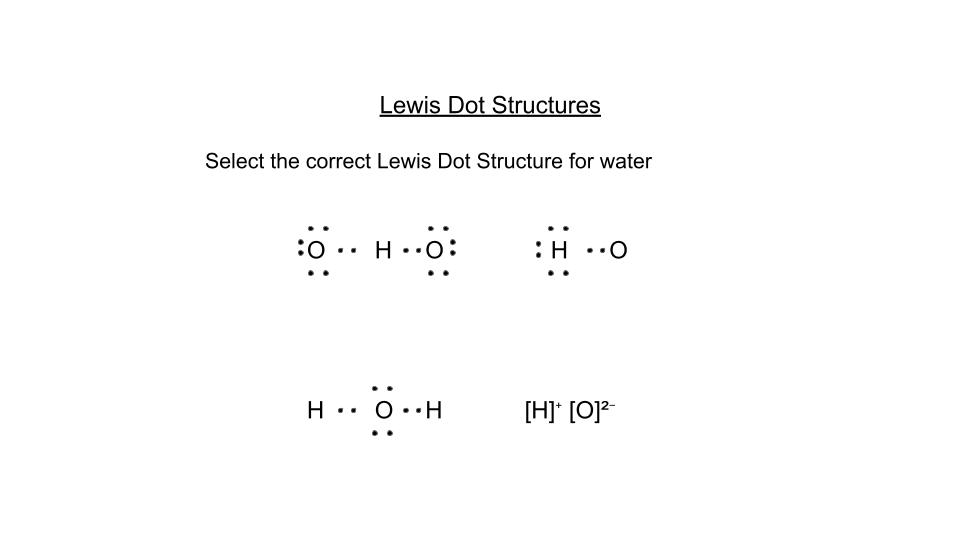
Review for Lewis Dot Structures, Naming Ionic and Covalent Compounds, and VSEPR Theory
Required
12
Required
10
Required
1
Required
1
Required
1
Required
1