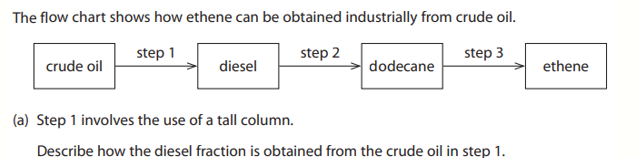
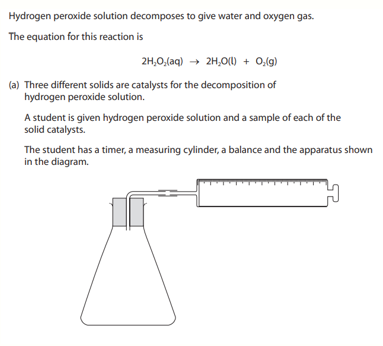
A teacher drops a bottle containing sodium chloride. The bottle breaks when it hits the floor. The teacher sweeps up the mixture of sodium chloride and glass. Describe how the teacher can obtain a pure, dry sample of sodium chloride from the mixture (4 marks)