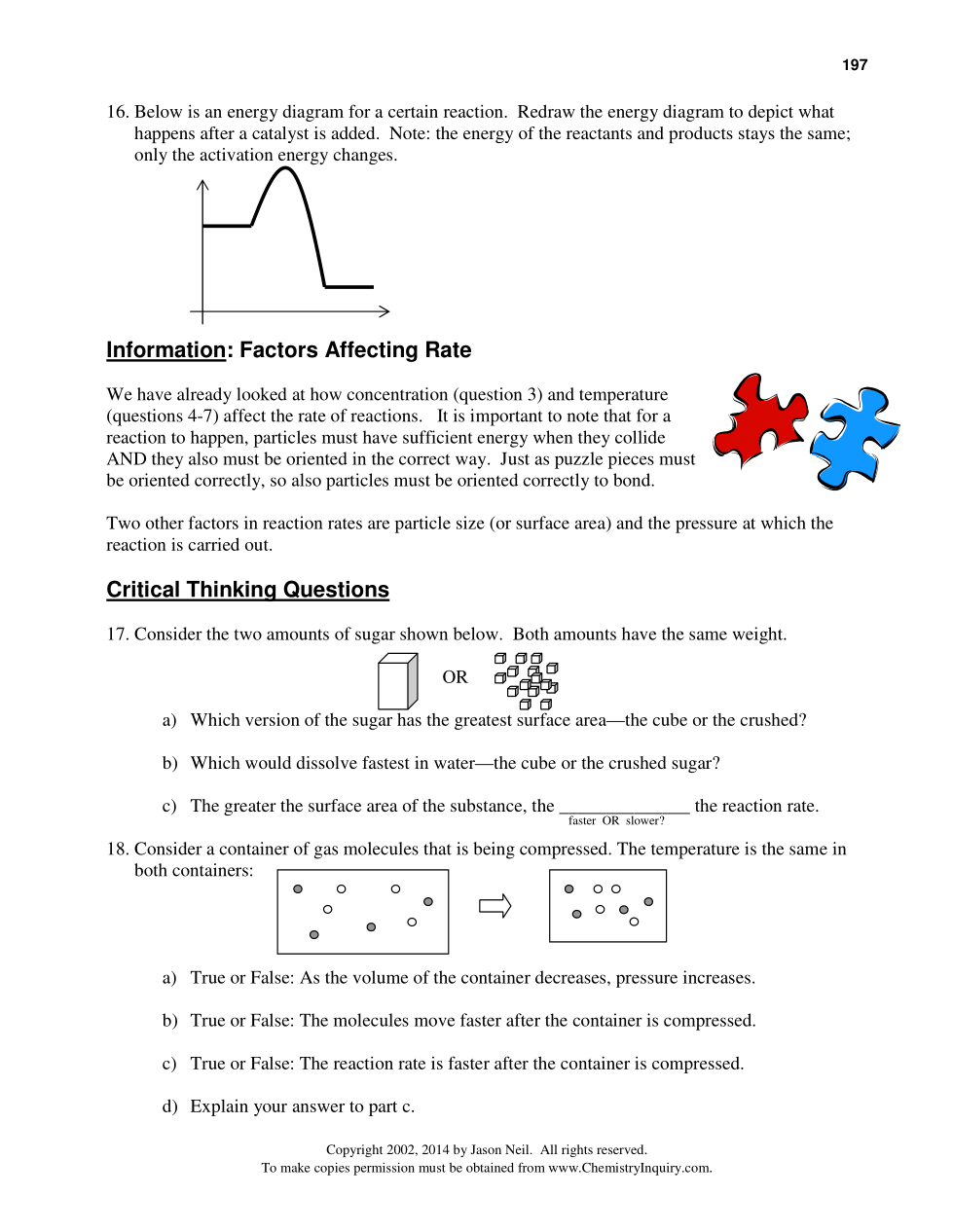
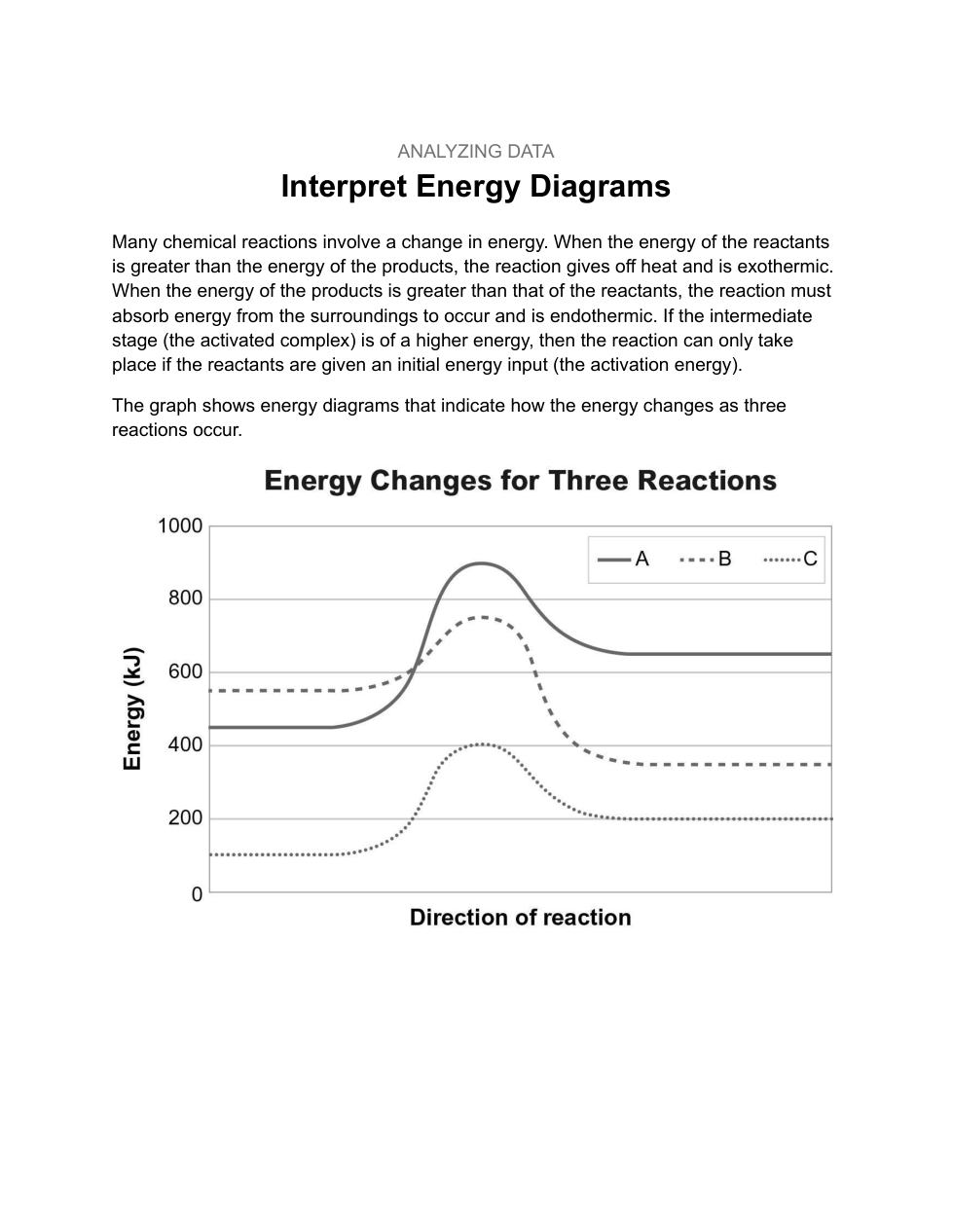
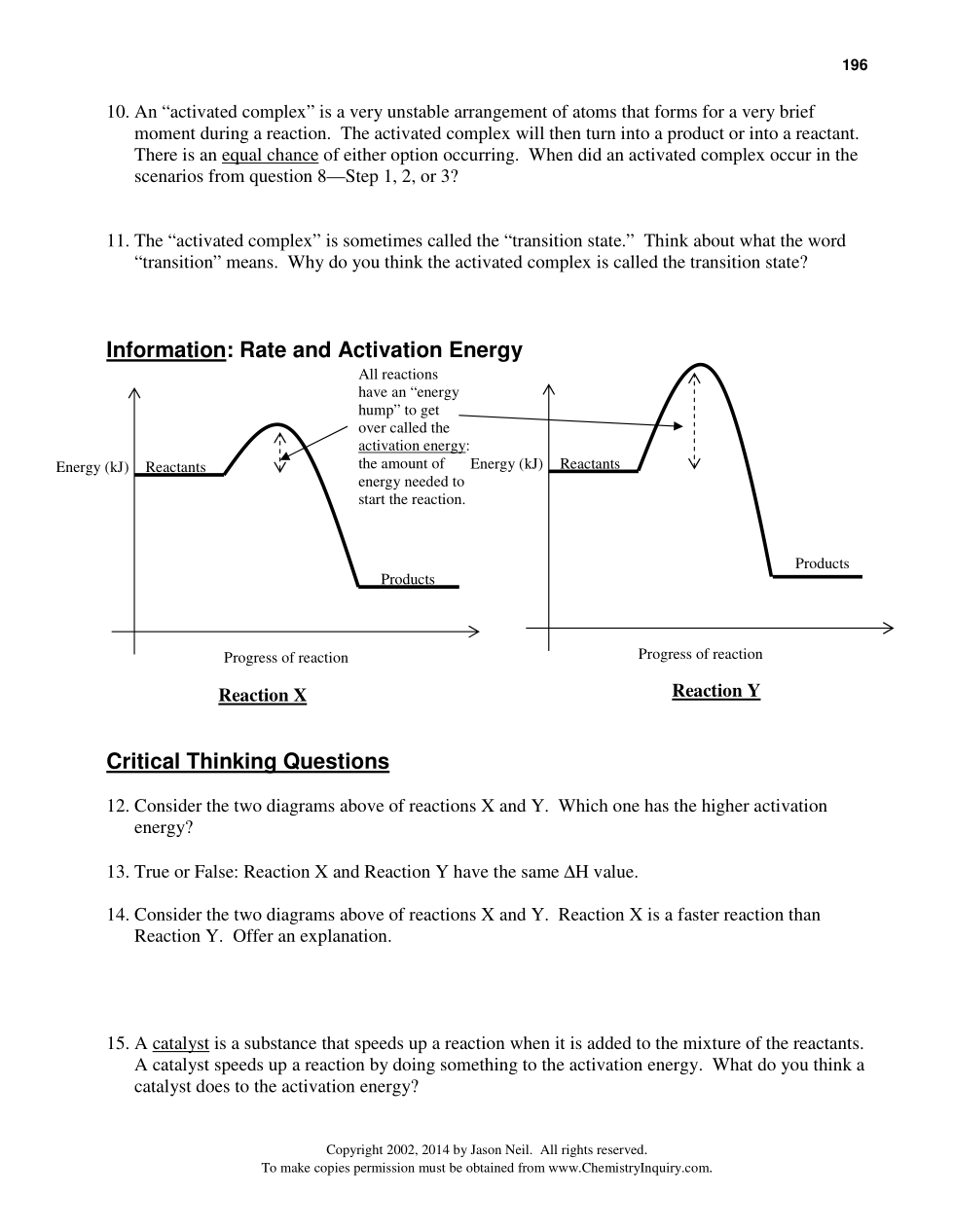
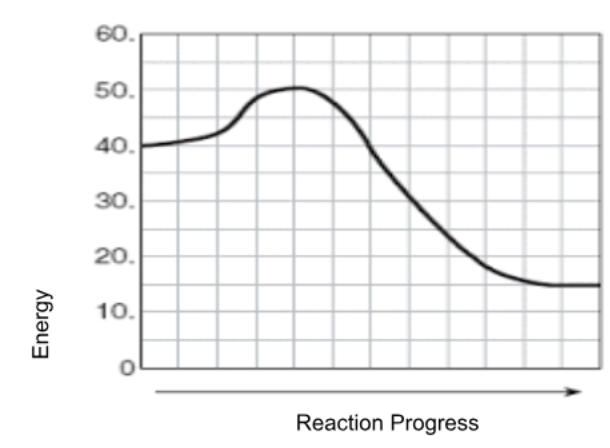
A scientist is testing a sample of a compound, Substance X, for its suitability for use as a fuel. The scientist finds that the sample will burn in air, reacting with oxygen to give off heat. The energy of the reactants is calculated as about 600 kJ, while the energy of the products is about 500 kJ. The activation energy is estimated to be about 100 kJ. Sketch a solid line on the graph to represent the energy changes for this reaction.